We are pioneering the development of PROTACs as an innovative strategy against Leishmania infections by selectively degrading trypanothione reductase (TR), a key enzyme essential for maintaining the parasite’s redox homeostasis. TR is unique to trypanosomatids and has no human homolog, making it an ideal and safe target for selective degradation. By leveraging the cellular ubiquitin–proteasome machinery, these bifunctional molecules enable event-driven elimination of TR, rather than simple inhibition, thus potentially overcoming drug resistance and minimizing host toxicity. This approach opens a new avenue for antiparasitic drug discovery based on targeted degradation (ACS Bio Med Chem Au, 2023, 3, 32–45).
Projects
TARGETED DEGRADATION TECHNOLOGIES
One of our group’s main research focuses is the development of small-molecule degraders for targets traditionally considered “undruggable.” Unlike conventional inhibition- or occupancy-driven approaches, these strategies harness the cell’s intrinsic quality-control systems to eliminate disease-causing molecules. To promote targeted protein degradation, we are advancing several event-driven technologies, including PROteolysis TArgeting Chimeras (PROTACs), Hydrophobic Tags (HyTs), and AUTophagy TArgeting Chimeras (AUTOTACs), each engaging distinct degradative pathways. In parallel, we are exploring RIBOnuclease TArgeting Chimeras (RIBOTACs), which extend this concept to RNA targets, enabling selective degradation of pathogenic RNAs.
PROTEOLYSIS TARGETING CHIMERAS (PROTACs)
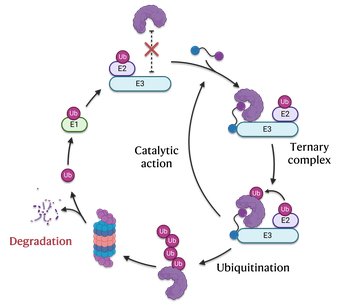
PROTACs are heterobifunctional small molecules that induce the selective degradation of target proteins by hijacking the ubiquitin–proteasome system. They consist of a ligand for the protein of interest (POI) linked to a ligand for an E3 ubiquitin ligase through an optimized chemical linker. By bringing the POI into close proximity with the E3 ligase, PROTACs facilitate its ubiquitination and subsequent proteasomal degradation, thereby achieving an event-driven rather than occupancy-driven pharmacological effect. This catalytic mechanism allows for sustained target depletion at sub-stoichiometric doses and the possibility to drug previously intractable proteins. Since their first demonstration by Crews and Deshaies (Sakamoto et al., Proc. Natl. Acad. Sci. USA, 2001, 98, 8554–8559) and the seminal expansion of the concept by the Ciulli and Bradner groups (Bondeson et al., Nat. Chem. Biol., 2015, 11, 611–617), PROTACs have transformed modern drug discovery by redefining how small molecules can modulate protein function.(as discussed in our perspective J. Med. Chem. 2022, 65, 14, 9507–9530).
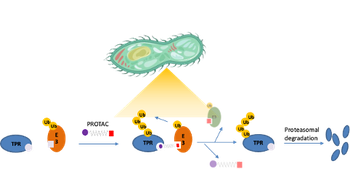
1) LEISHMANIASIS

2) FLAVIVIRUS
Flaviviruses are vector-borne RNA viruses causing severe diseases, including epidemics of dengue and West Nile Virus (WNV), and the recent outbreak of Zika virus (Pierson, T. C.; Diamond, M. S. Nat Microbiol. 2020, 5(6): 796–812). Measure to control and cure flavivirus-induced infections are largely underestimated. To mitigate current health burden and limit future outbreaks, drugmakers need to be ready and avoid that the spread of flaviviruses will materialize, in the worst-case scenario, in a pandemic. Currently, there is neither vaccine nor drug to treat flavivirus infection, making drug-discovery efforts extremely urgent. Despite different families of small molecules or peptidomimetic inhibitors have been reported, none of them reached clinical phases (Voss, S.; Nitsche, C. RSC Med. Chem., 2021, 12, 1262).
The NS2B-NS3 protease is essential for the replication of flaviviruses and, importantly, is conserved among WNV, Dengue and Zika viruses. Therefore, our final PROTACs should possess pan-flaviviral activity and might represent a new avenue to target flaviviruses.

3) NEUROINFLAMMATION
Increasing evidence suggests that Alzheimer Disease (AD) pathogenesis is a complex multifactorial event, which includes β-amyloid aggregation, neurofibrillary tangle formation, and neuroinflammation (Genome Med. 2023;15(1):6. Published 2023 Jan 26). Neuroinflammation is one of the cardinal features of AD and refers to a brain specific and chronic inflammation-like response that leads to neuronal death. Central and peripheral immune systems trigger an innate immune response characterized by release of inflammatory mediators, which contribute to AD progression and severity. Thus, modulating crucial neuroinflammatory targets is a strategy under intensive investigation in AD drug discovery. On this basis, in this project, we have turned our attention to PROTACs for key neuroinflammatory targets.
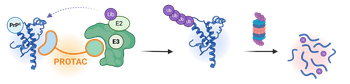
4) PRION DISEASES
Prion diseases are fatal neurodegenerative disorders marked by the accumulation of misfolded prion protein (PrPSc) and the consequent collapse of neuronal proteostasis. To date, no disease-modifying therapies exist, and current treatments are purely symptomatic. In this project, we apply the PROTAC strategy to induce the selective degradation of native cellular prion (PrPC)by repurposing a known PrP-binding molecule as the targeting ligand. This design aims to clear pathogenic PrP species rather than merely inhibit their formation, restoring proteostasis and neuronal health.

RIBONUCLEASE TARGETING CHIMERAs (RIBOTACs)
Targeting RNA with small molecules represents one of the most exciting frontiers in modern medicinal chemistry. While current RNA-based therapies—such as antisense oligonucleotides, siRNAs, and CRISPR systems—face challenges including poor cellular uptake, limited tissue specificity, and toxicity, small molecules offer a promising alternative. Among emerging RNA-targeting strategies, RIBOnuclease TArgeting Chimeras (RIBOTACs) stand out for their ability to induce the selective degradation of disease-related RNAs. RIBOTACs are bifunctional small molecules that link an RNA-binding warhead to a recruiter of the endogenous RNase L enzyme, forming a ternary complex that catalyzes RNA cleavage. Supported by the National Recovery and Resilience Plan (NRRP), this project focuses on developing RIBOTACs against pre-miR-21, a proto-oncogenic microRNA involved in fibrosis, inflammation, and neurodegeneration (Hargrove, Chem. Commun., 2020, 56, 14744–14756; Childs-Disney et al., Nat. Rev. Drug Discov., 2022, 21, 736–762*).
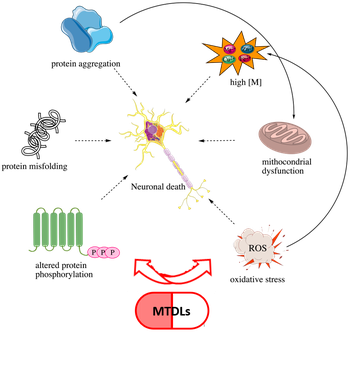
MTDLs FOR NEURODEGENERATIVE DISEASES
Neurodegenerative diseases arise from complex, interconnected molecular dysfunctions that cannot be addressed by single-target drugs. The multi-target-directed ligand (MTDL) strategy embraces a polypharmacological approach, enabling the simultaneous modulation of multiple pathways involved in neurodegeneration onset and progression. Our group designs and synthesizes MTDLs to tackle key mechanisms in Alzheimer’s disease (AD)—including cholinergic dysfunction, oxidative stress, and neuroinflammation. With only palliative treatments currently available and AD cases projected to rise sharply by 2050, the need for innovative, disease-modifying agents is urgent. Academic research thus plays a critical role in advancing this frontier (J. Med. Chem., “Multi-target-directed ligands to combat neurodegenerative diseases”).

SUSTAINABLE DRUG DISCOVERY
The research and development of new potential drug candidates require huge expenses and efforts which are eventually mirrored in the final price of the drugs on the market. Access to medicines, including availability and affordability is a major global public health threat for all the therapeutic areas. It is a matter of special concern for those diseases affecting low-income populations (i.e. poverty-related disease as NTD) but also for Alzheimer’s Disease, which is expected to affect large populations in the developing countries. Through a Sustainable Drug Discovery approach, we aim to develop potential treatments that are affordable and globally accessible. In this context, an enlightening possibility is represented by the valorization of food byproduct material (Chem. Soc. Rev., 2021, 50, 11191-11207), such as the cashew-nutshell liquid (CNSL). CNSL components (i.e. anacardic acid, cardanol, cardol) are bioactive phenolic compounds with a linear chain of different unsaturation degrees. Thanks to their peculiar amphiphilic structure and their innate biological activities, CNSL components are particularly versatile for the functionalization and the design of MTDLs for diverse diseases (e.g., ChemMedChem 2019, 14, 621 and J. Med. Chem. 2021, 64, 4972−499).
How to reach us
Contatti
Ph.D. Student Office
+39 051 20 9 9717