BACTERIAL FILTRATION EFFICIENCY TEST
The design of the BFE apparatus requires several specific instruments, as indicated in the layout of the system developed according to the standard norm indications. Due to the full lockdown in the pandemic outbreak in spring 2020 and the short time available required by the urgent needs, not all such pieces of equipment were on the market. Therefore, we were forced to adapt components already presents in our laboratories, spare parts, and even pieces disassembled from other apparatuses. Other components were generously donated by companies, citizens or other University of Bologna Departments. Over the subsequent months and for the whole 2020, the initial version of the apparatus has been improved.
- The first version of the BFE apparatus, illustrated in the Figure was installed in a disused surgery room at the University hospital (St. Orsola), in order to exploit of the sterile conditions required for the tests and for a higher safety of the workers.
- The air, coming from the sterile room, enters in a transparent, poly methyl methacrylate (PMMA) tube, with an internal diameter of 80 mm and flanged at both ends. [1]
- At half of the tube height (700 mm), a pressure driven nebulizer (Collison single-jet nebulizer by CHT technologies) nebulizes a Stafilococcus aureus solution.
- The two-phase mixture produced in the cylinder reaches the bottom where the mask sample, appropriately cut of the size requested by the standard norm, is placed between two flanges.
- The bottom flange was connected to the underneath impactor by a connection specifically designed by the software Autodesk Inventor 2019 and produced by a 3D printer (Prusa i3 MK3S+).
- The bacteria containing droplets permeating through the mask sample are collected in an Andersen 6-stages impactor, generously donated by Cavazza Anna SAS (Bologna, Italy). The impactor is able to collect droplets of different sizes, with diameter down to 0.65 µm.
- To avoid any possible diffusion of bacteria in the environment, smaller contaminated droplets are collected in a vacuum trap, preceded by a glass, water cooled condenser. To this aim, two different configurations have been considered, the first one, using tap water available in the operating theatre, while later, a mini-chiller has been implemented in the system, in order to reduce the water consumption a to ensure proper cooling also during warmest periods.
- The droplet-free air finally reaches a flow indicator (Bronkhorst el-flow, range 0-100 L/min) generously donated by IMA SpA (Ozzano Emilia (Bologna), Italy), a valve for flow regulation and a vacuum pump that provides the required driving force for the air flow. [2]
- The aerosol used during the tests consists of a bacterial suspension of Staphylococcus aureus ATCC6538, which is prepared at a concentration of 5 · 105 CFU colony forming units (CFU) /mL by diluting 1/7000 a culture with 1.8 McFarland turbidity standard in modified peptone water (peptone 5 g/L, NaCl 5 g/L). This inoculum preparation allows to test approximately 1.7 - 3 x 103 CFU per test (as suggested by the standard norm).
- The bacterial aerosol is transported inside the tube thanks to the activity of a vacuum pump placed at the end of the test line with a flow rate of 28.3 L/min. This will force the aerosol to pass through the mask, and the bacteria not retained by the mask are collected in the stages of the impactor, where 6 horse blood agar petri dishes are placed, one for each impactor stage.
- The bacterial colonies (CFU) that will grow on these plates are counted after a 24-48 h incubation at 37 °C.
- The number of CFU grown on the plates are used to calculate the BFE by comparing the total CFU obtained by testing 5 masks with the number of CFU present in the bacterial aerosol (see e.g., Figure 2d).
- The mean value and the standard deviation have been calculated using the CFU data from the 5 different specimens obtained by 5 different masks analyzed. To assess the absence of environmental contamination, a negative control was also performed, by letting air flow through the impactor, in the absence of both the mask and the nebulized bacterial aerosol starting from the suspension of the inoculum.
Notes
[1] Pipe length and material: The normative requires a glass tube with an internal diameter of 80 mm and a height of 600 mm, from the top of which the aerosol enters. No interferences between the plastic tube and the aerosol bacteria were observed, as shown by all the negative control run performed during the entire period of investigation that demonstrated the absence of contamination in the tube. However, after the emergency time and by the end of the lockdown period, the plastic PMMA tube was replaced by the standard glassy tube, prepared strictly according to the standard norm and no differences were observed in the results obtained.
[2] Air flow humidity: Interestingly, the standard norm contains specific indications about the conditioning protocol for the specimens (room T at 85% R.H. for at least 4 h), while there is no mention about the humidity of the feed air used for the test, even though that is very relevant in the case of multiphase transport of air containing water droplets. In our experiments the dry air avilable in t he sterile room (30% R.H.) was humidified before entering the tube, by saturation with demineralized water. Such procedure ensured the evaporation of the droplets before impacting the mask specimen. The temperature and the R.H. in the cylinder were measured by a digital thermo-hygrometer. See the section Droplet size and air humidification.
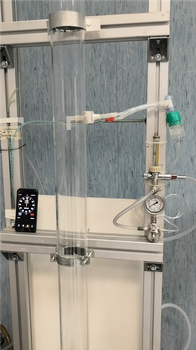
A picture of the upper part of the apparatus depicting part of the column and the aerosol injection via the nebulizer

Impactor assembled

The six stages of the impactor highlighting the decreasing size of the pores and the sealing o-rings

The colony count: on top results of a test carried out without a mask. On the bottom, the results of a test performed with a masks having a bacterial filtration efficiency higher than 98%.
You can see the higher number of colonies in the top row and the small number of colonies in the bottom row
