Tests
Test required to certify surgical masks
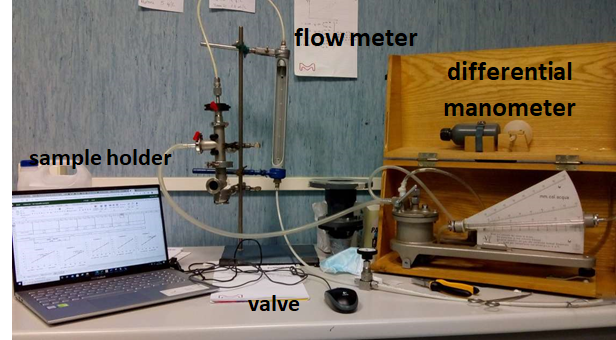
BREATHABILITY
It is a measure of the respiratory resistance provided by the mask, and thus the effort required by the mask wearer in order to breath with conventional flow rates. Taking advantage of the uniformity of the surgical mask, the tests are required to be executed on a representative section, a circular sample of 25 mm of diameter, in which the pressure drop need to be measured when impacted by 8 L/min air flow;
Such test is of paramount importance for a surgical mask because, unlike respirators (personal protective equipment, PPE), there are no specific element that provide a good adhesion with the face, and thus the sealing of the equipment is poor. Hence, if the resistance to the air flow caused by the masks is too high, a fraction of the air passes through the boundaries instead that through the mask, reducing the protection offered by the device.

BACTERIAL FILTRATION EFFICIENCY (BFE)
The test represents the direct evaluation of the effectiveness of the filtering device, as it measures the number of liquid droplets containing bacteria that permeated through a circular sample (80 mm diameter) fed by two-phase mixture (gas + liquid droplets containing bacteria). The efficiency is calculated from the ratio between the number of droplets (i.e. bacteria) permeated vs. those fed to the sample mask, being the latter directly measured from a “blank” experiment (i.e. with no filtering mask).

SPLASH TEST
Resistance against penetration by synthetic blood (also called “splash test”)
The test aims to verify the protection offered to the operator wearing a surgical mask of a specific type (IIR) with respect to a blood squirt, which may occur during a surgical operation. Therefore, the penetration of a certain fixed volume of blood simulant impacting frontally the sample at known velocity, is evaluated. The blood should not wet the internal layer of the masks, and thus to be in contact with the operator lips and nose
BIOBURDEN
Sterilization of health care products (“Bioburden”):
The test aims to evaluate quantitatively the number of microorganisms present on the mask before wearing it for the first time. Although it is crucial for the final evaluation of a surgical mask to be made available in the masked and used by the population, it may be considered of a minor relevance with respect to the previous tests when a mask prototype is first evaluated to determine its performances. To some extent, bioburden tests evaluate the cleanliness of the production line and of the effectiveness of the sterilization and packaging lines after the production