Functional analysis

Volumetric gas absorption (Sievert) apparatus
A Sievert’s apparatus serves as a pivotal tool in materials research, enabling the comprehensive study of powdered alloys thermodynamic and kinetic properties. It consists of several chambers of known volume interconnected through manual and software-controlled valves to facilitate the controlled flow of hydrogen gas. Temperature variations and pressure levels are monitored by integrating thermocouples and pressure gauges.
The central objective of a volumetric experiment performed on a Sievert's apparatus is to expose a sample to a predetermined hydrogen pressure at a fixed temperature, observing the alloy response over time. Notably, hydride formation is associated with a specific constant equilibrium pressure within a defined range of hydrogen concentration. When the hydrogen pressure overcomes this critical threshold, hydrogen absorption may occur. Conversely, exposing a filled metal hydride below equilibrium conditions results in the release of hydrogen gas.
Concerning the context of hydrogen storage applications, volumetric measurements hold profound importance in discerning essential parameters. These include the rate constant governing the speed of sorption processes, the activation energy required for these transformations, as well as the enthalpy and entropy associated with hydride formation and decomposition. These parameters are intrinsically linked to the macroscopic pressure-temperature parameters through the Van’t Hoff equation, offering insights into the underlying thermodynamics. By performing kinetic measurements and outlining the so-called Pressure-Composition-Isotherms (PCIs), which consist of a series of partial kinetics near the equilibrium pressure, it is possible to unveil the aforementioned parameters, gathering precious information on the material sorption behaviours.
Volumetric measurements integrated with morphological and structural analyses result in a deeper comprehension of the optimal adjustment of the material's stoichiometry. This adjustment enables the material to align with the operational temperature and pressure conditions required for solid-state storage applications.
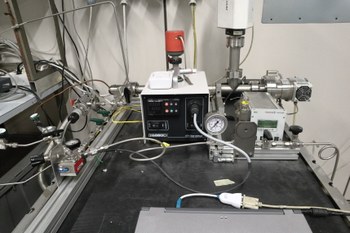
High-pressure Thermo/Photoreactor
The reactor is equipped with CO2, CO, H2 gases, in order to investigate the catalytic efficiency of thermo- photo-catalysts towards gas-phase reactions, such as syngas (Co/H2) conversion to methanol. A quartz window allows for AM 1.5 G illumination, while the sample holder (powder/pellets) can be heated up to 300°C. The cell is able to operate in a pressure range betwee 1 mbar and 28 bar. The reactor is coupled to a high-vacuum line equipped with a mass spectrometer, allowing for in-line detection of gaseous reaction products.

Micro Gas Chromatography
Micro gas chromatography (μ-GC) system equipped with two columns (Molesieve 5A + PoraPlot Q) for real-time sampling and analysis of gas products from electrochemical cells used in water splitting and CO₂ reduction. Its miniaturized design enables rapid, high-resolution separation and detection of gaseous products like H₂, CO, O₂, and light hydrocarbons, generated at low concentrations during electrochemical reactions. The analytical tool is directly coupled with bulk and flow PEC cells for headspace sampling and real-time calculation of Faradic efficiency.