Solid state hydrogen storage
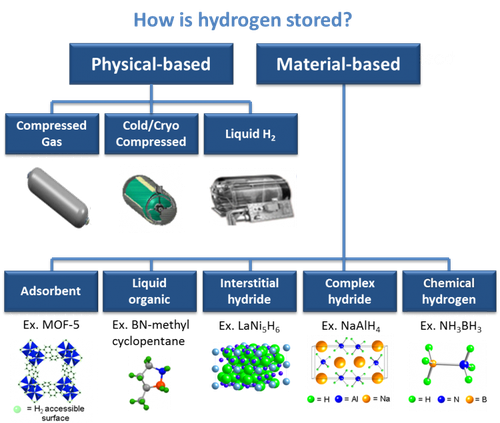
Nowadays, the most common way of storing hydrogen is in pressure tanks, like most of the gases used in industry.In order to store a significant amount of hydrogen, tanks have to sustain pressures of hundreds of atmospheres. This has drawbacks in terms of costs, weight and sometimes safety. All these reasons prevents hydrogen from having widespread usage as a way to store and transport energy. The ideal storage system should be light, cheap and should work at pressure and temperature close to ambient condition.
Magnesium is a light metal which forms a hydride with a considerable gravimetric density (8% in hydrogen weight). Unfortunately, the chemical reaction is slightly too exothermic . This means that getting the hydrogen back in the gas form requires thermal energy. This makes storing hydrogen in magnesium very wasteful in terms of energy costs.
Other compounds which are under investigation are the so-called intermetallic compounds. This class has basic chemical formula ABx, where A is an element which form strong bonds with hydrogen, whereas B does not.The chemical reaction with hydrogen has the right amount of exothermicity. Therefore, their working pressure at room temperature is around 1 atmosphere.Chemical doping with other elements plays an important role in tuning the properties of the material to best suit the application it is intended for.Since this compounds are made of heavier elements than Mg, the gravimetric density they can reach is lower, about 2% in hydrogen weight.Some well-known examples which belong to this class of compounds are TiFe, TiMn2, LaNi5.
Intermetallic compounds with base formula AB5 currently find application as anodes in rechargeable NiMH batteries.