Publications
2025

Operando x-ray absorption spectroscopy unveils light-driven redox dynamics at the semiconductor/cocatalyst interface
Sci. Adv.11,eadx8089(2025).DOI:10.1126/sciadv.adx8089
Cobalt-based mixed oxides are widely studied as oxygen evolution reaction (OER) catalysts, yet their role as photoelectrochemical cocatalysts remains debated due to scarce operando studies probing irradiation-induced structural changes. Here, we unveil redox dynamics of cobalt-iron oxide (CoFeOx) cocatalysts in semiconductor photoanodes for solar water splitting. By combining operando x-ray absorption spectroscopy (XAS) with fixed-energy x-ray absorption voltammetry (FEXRAV) at semiconductor/cocatalyst interfaces, we provide an element-selective probe of Co oxidation states under dark and illuminated conditions. Our results reveal a previously unrecognized interfacial Co state, highlighting interface structure’s role in tuning catalytic activity. We observe light-induced reduction in oxidation state and cathodic shift in Co redox potentials, offering insights into hole transfer and catalytic behavior. Identification of a specific photocatalytic cycle, distinct from dark-state electrocatalysis, advances understanding of how light modulates rate-determining steps in OER. These findings underscore the power of operando x-ray techniques in elucidating interfacial charge transfer and guiding design of more efficient photoelectrochemical systems.

Elucidating Ti Dopant Effects in Hematite Photoanodes via High-Throughput Combinatorial Screening
J. Mater. Chem. A, 2025, Accepted Manuscript
Hematite (α-Fe₂O₃) represents a promising candidate for photoelectrochemical water splitting, but its practical application is hindered by poor charge transport and sluggish surface kinetics. Despite extensive research on titanium doping as a performance enhancer, the underlying mechanisms remain debated, with widely varying optimal concentrations reported across different fabrication techniques. Here, we demonstrate how high-throughput combinatorial approaches can rapidly elucidate complex structure-function relationships that would be challenging to identify through conventional methods. We first optimize titanium content using a concentration gradient sample, then leverage this optimal composition to create two complementary sample types: one with titanium co-sputtered throughout the hematite film and another with titanium sequentially deposited as an overlayer. By subjecting both sample types to similar temperature gradients, we create a powerful comparative framework that reveals how the same elements behave differently based on initial spatial distribution, yet converge toward similar structures at higher temperatures. This parallel temperature evolution allows us to precisely identify the conditions where performance dramatically changes and target these specific regions for in-depth structural characterization. Our findings reveal that Ti enhances photoactivity through two pathways: modifying surface properties to improve charge transfer efficiency and acting as a substitutional dopant to increase bulk charge separation. Structural analysis confirms titanium incorporates into hematite up to a thermodynamic solubility limit of approximately 4-5 at.%, with excess titanium segregating to form surface TiO₂ phases that become detrimental beyond optimal thickness. This comparative gradient approach represents a powerful strategy for simultaneously optimizing materials while unlocking fundamental mechanistic insights that are difficult to gain by means of conventional single-parameter studies.

Hybrid Molecular Photoanodes for Water Oxidation Based on Electropolymerized Cu Macrocyclic Complexes on BiVO4-WO3
Adv. Energy Mater. (2025): e00253. https://doi.org/10.1002/aenm.202500253
The conversion of sunlight into chemical energy provides a sustainable alternative to fossil fuels that can significantly contribute to the mitigation of climate change. In this regard, water splitting with sunlight using semiconductors coupled with redox catalysts emerges as a potential pathway to generate green hydrogen. Here, the performance of molecular hybrid materials composed of inorganic semiconductors, WO3-BiVO4, combined with molecular water oxidation catalysts based on Cu macrocyclic complexes is described. It is found that the charge transfer from BiVO4 to the molecular catalyst occurs on a similar time scale to the direct interfacial hole transfer to water, with a concomitant 62% decrease in the recombination rate because recombination centers are passivated upon deposition of the Cu molecular catalyst on the WO3-BiVO4 junction. Overall, this results in an improvement of the photocurrent as well as long-term stability of the new hybrid materials generated.

Synthesis and Hydrogen Storage Properties of Mg-Based Complex Hydrides with Multiple Transition Metal Elements
ACS Appl. Energy Mater. 2025, 8, 8, 4993–5003
Mg2TMHn complex hydrides, where TM represents various combinations of transition metals, were synthesized by reactive ball milling of Mg and TM powders under H2 pressure. TM was an equimolar mixture of three (Fe, Co, and Ni), four (Mn, Fe, Co, and Ni), or five (Cr, Mn, Fe, Co, and Ni) elements. The Mg/TM ratio was either 2:1 or 3:1. For 2:1 samples, a single fcc hydride phase Mg2TMHn with a K2PtCl6-type structure was detected by X-ray diffraction along with a residual, unreacted metal phase. By contrast, in samples where the Mg/TM ratio was 3:1, the tetragonal MgH2 hydride was also observed. The formation of Mg3TMHn complex hydrides, previously reported for TM = Cr and Mn under high-pressure conditions, was not detected. The maximum hydrogen content in the as-milled state was about 5 wt% for samples with a 3:1 Mg/TM ratio as determined by temperature-programmed desorption. The as-milled hydrides exhibited similar onset temperatures for desorption independently of the TM composition, suggesting no destabilization induced by elements like Mn and Cr that are known to form only unstable, high-pressure hydrides. The reversible hydrogen storage, investigated by pressure–composition isotherms in a Sieverts-type apparatus, arises from both the Mg-MgH2 and the Mg2TM-Mg2TMHn transformations. Within the 0.1–20 bar and 285–320 °C window, the samples with a 3:1 Mg/TM ratio exhibit a reversible gravimetric capacity in the 3.7–4.2 wt% range depending on TM composition, while those with a 2:1 ratio are in the 3.0–3.2 wt% range. The decreased reversible capacity compared to the initial hydrogen content was associated with the phase segregation of the transition metals, particularly Cr and Mn, which was highlighted by X-ray diffraction and transmission electron microscopy with nanoscale microanalysis.

Tuning TiFe1–xNix Hydride Thermodynamics through Compositional Tailoring
ACS Appl. Energy Mater. 2025, 8, 4, 2135–2144
In this study, we investigate how structural modifications induced by Fe substitution with Ni in the TiFe intermetallic alloy affect the thermodynamics of hydride formation and decomposition. The primary goal of substituting Fe with Ni was to reduce the plateau pressure of TiFe, a crucial parameter for reversible solid-state hydrogen storage applications under near-ambient conditions (below 150 °C and 50 bar). Alloy compositions TiFe1–xNix with x ≤ 0.30 were synthesized by arc melting. The structural and morphological properties were characterized using powder X-ray diffraction and scanning electron microscopy with energy-dispersive X-ray spectroscopy. The thermodynamic properties were investigated through volumetric measurements using a Sieverts’ apparatus and calorimetric analysis with a high-pressure differential scanning calorimeter. We show that Ni incorporation effectively lowers the plateau pressure, stabilizing the hydride thermodynamics due to a more negative enthalpy of hydride formation. Moreover, the entropy of hydride formation increases with the Ni content, resulting in a linear correlation between the enthalpy and entropy values determined at different compositions. The enthalpy–entropy compensation effect was analyzed to determine whether it arises from statistical artifacts or is genuine to the system, as our findings suggest.
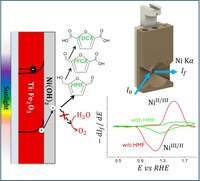
Nickel-Based Cocatalysts on Titanium-Doped Hematite Empower Direct Photoelectrochemical Valorisation of 5-Hydroxymethylfurfural
ChemSusChem, early view
The photoelectrochemical oxidation of 5-hydroxymethylfurfural (HMF), a biomass-derived intermediate, to 2,5-furandicarboxylic acid (FDCA), a key building block for industrial applications, is a well-studied anodic reaction. This photoelectrochemical (PEC) conversion typically requires an electron mediator, such as TEMPO, regardless of the semiconductor used. Various electrocatalysts can also perform this reaction electrochemically, without additional organic species in the electrolyte. In this study, Ti-doped hematite (Ti:Fe2O3) photoanodes were employed for the HMF photoelectrochemical conversion at the anodic side of a two-compartments PEC cell. To avoid the need of an electron mediator, nickel-based electrocatalysts were deposited on the electrode′s surface. The Ni(OH)2-electrodeposited (Ti:Fe2O3−Ni) and the NiMo-sputtered Ti:Fe2O3 photoanodes (Ti:Fe2O3−NiMo) were characterised and tested for the HMF oxidation in 0.1 M NaOH (pH 13) electrolyte. Partial HMF photoelectrochemical conversion to FDCA was achieved, pointing out the beneficial effect of Ni-based cocatalyst in shifting the selectivity towards the di-carboxylic acid. Fixed Energy X-ray Absorption Voltammetry (FEXRAV) and X-ray Absorption Near-Edge Structure (XANES) measurements were conducted to investigate the interaction between HMF and the two deposited electrocatalysts. These techniques offered valuable insights into the oxidation mechanism, which were further validated using a rate deconvolution procedure.
2024
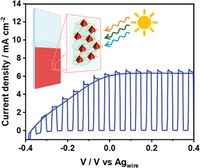
Highly Efficient Photoanodic Material: Utilizing Dihydrolipoic Acid-Functionalized CuInS2 Quantum Dots in Photoelectrochemical Cells
Advanced Optical Materials, Volume12, Issue19, July 5, 2024, 2400259
Copper indium sulfide quantum dots (CIS QDs) possess the desired optical properties to act as photoanodic material in photoelectrochemical cells, like a high molar absorption coefficient over the entire visible spectrum and long exciton lifetimes. The already reported procedures that utilize photoanodes based on such nanoparticles, however, exploit harsh conditions or utilize non-scalable, expensive, and low-yield syntheses. Here, the construction of CIS QDs adsorbed onto TiO2/FTO photoanodes (FTO = fluorine-doped tin oxide) with a process aimed at avoiding these issues is proposed. In particular, the employment of dihydrolipoic acid as the ligand allows an easy and cost-effective functionalization. CdS layers are deposited onto the nanoparticles to enhance the photoelectrochemical properties. Full characterization of the steady–state and transient photoelectrochemical properties of the electrodes is performed to gain information on the interfacial dynamics among the different components of the electrode. Maximum IPCEs of the order of 50% and a spectral sensitization extended up to 700 nm are obtained in the optimized conditions.
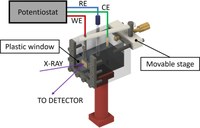
Operando double-edge high-resolution X-ray absorption spectroscopy study of BiVO4 photoanodes
Journal of Synchrotron radiation, Volume 31| Part 3| May 2024| Pages 464-468
High energy resolution fluorescence detected X-ray absorption spectroscopy is a powerful method for probing the electronic structure of functional materials. The X-ray penetration depth and photon-in/photon-out nature of the method allow operando experiments to be performed, in particular in electrochemical cells. Here, operando high-resolution X-ray absorption measurements of a BiVO4 photoanode are reported, simultaneously probing the local electronic states of both cations. Small but significant variations of the spectral lineshapes induced by the applied potential were observed and an explanation in terms of the occupation of electronic states at or near the band edges is proposed.
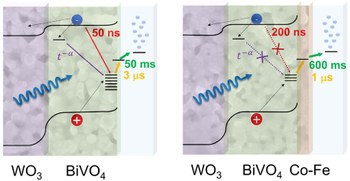
Impact of Co–Fe Overlayers on Charge Carrier Dynamics at WO3/BiVO4 Heterojunctions: A Picosecond-to-Second Spectroscopic Analysis
ACS Energy Lett. 2024, 9, XXX, 2193–2200
Surface modification with CoFe-based overlayers has been widely studied to improve the performance of WO3/BiVO4 photoanodes for photoelectrochemical water oxidation because such overlayers can increase the photocurrent and shift the onset potential to more favorable values. Herein, we present a transient absorption spectroscopic analysis of WO3/BiVO4 photoanodes coated with cobalt iron oxide or cobalt iron Prussian blue overlayers, designed to establish the underlying mechanisms for these enhancements on the picosecond-to-second time scale. The data reveal that the overlayer suppresses recombination of trapped holes in BiVO4, with free and trapped electrons, and accepts photogenerated holes. These results show that the observed boost in efficiency for water oxidation can be explained by the dual role of the overlayer in inhibiting charge recombination and enhancing charge extraction.
2023
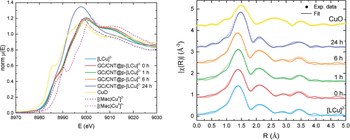
Robust Molecular Anodes for Electrocatalytic Water Oxidation Based on Electropolymerized Molecular Cu Complexes
Advanced materials, Volume 36, Issue7, February 15, 2024, 2308392
A multistep synthesis of a new tetra-amidate macrocyclic ligand functionalized with alkyl-thiophene moieties, 15,15-bis(6-(thiophen-3-yl)hexyl)-8,13-dihydro-5H-dibenzo[b,h][1,4,7,10]tetraazacyclotridecine-6,7,14,16(15H,17H)-tetraone, H4L, is reported. The reaction of the deprotonated ligand, L4−, and Cu(II) generates the complex [LCu]2−, that can be further oxidized to Cu(III) with iodine to generate [LCu]−. The H4L ligand and their Cu complexes have been thoroughly characterized by analytic and spectroscopic techniques (including X-ray Absorption Spectroscopy, XAS). Under oxidative conditions, the thiophene group of [LCu]2- complex polymerizes on the surface of graphitic electrodes (glassy carbon disks (GC), glassy carbon plates (GCp), carbon nanotubes (CNT) or graphite felts (GF)) generating highly stable thin films. With CNTs deposited on a GC by drop casting, we obtain hybrid molecular materials labeled as GC/CNT@p-[LCu]2−. The latter are characterized by electrochemical techniques that show their capacity to electrocatalytically oxidize water to dioxygen at neutral pH. These new molecular anodes achieve current densities in the range of 0.4 mA/cm2 at 1.30 V versus NHE with an onset overpotential at approx. 250 mV. Bulk electrolysis experiments show an excellent stability achieving TONs in the range of 7600 during 24 h with no apparent loss of catalytic activity and maintaining the molecular catalyst integrity, as evidenced by electrochemical techniques and XAS spectroscopy. Further with highly porous graphitic materials such as GF, we obtain TONs in the range of 11,000.
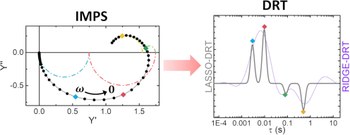
Distribution of Relaxation Times Based on Lasso Regression: A Tool for High-Resolution Analysis of IMPS Data in Photoelectrochemical Systems
J. Phys. Chem. C 2023, 127, 17, 7957–7964
Intensity-modulated photocurrent spectroscopy (IMPS) has been largely employed in semiconductor characterization for solar energy conversion devices to probe the operando behavior with widely available facilities. However, the implementation of IMPS data analysis to complex structures, whether based on the physical rate constant model (RCM) or the assumption-free distribution of relaxation times (DRT), is generally limited to a semi-quantitative description of the charge carrier kinetics of the system. In this study, a new algorithm for the analysis of IMPS data is developed, providing unprecedented time resolution to the investigation of μs to s charge carrier dynamics in semiconductor-based systems used in photoelectrochemistry and photovoltaics. The algorithm, based on the previously developed DRT analysis, is herein modified with a Lasso regression method and available to the reader free of charge. A validation of this new algorithm is performed on a α-Fe2O3 photoanode for photoelectrochemical water splitting, identified as a standard platform in the field, highlighting multiple potential-dependent charge transfer paths, otherwise hidden in the conventional IMPS data analysis.
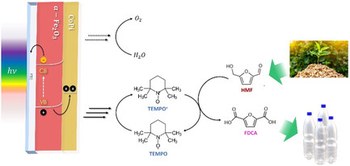
Photoelectrochemical Valorization of Biomass Derivatives with Hematite Photoanodes Modified by Cocatalysts
Sol. RRL, 2023, 2300205
The solar-driven oxidation of biomass to valuable chemicals is rising as a promising anodic reaction in photoelectrochemical cells, replacing the sluggish oxygen evolution reaction and improving the added value of the energy conversion process. Herein, the photooxidation of 5-hydroxymethylfurfural into furan dicarboxylic acid (FDCA) is performed in basic aqueous environment (borate buffer, pH 9.2), with the addition of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as redox mediator. Because of its good stability, cost-effectiveness, and nontoxicity, titanium-modified hematite (Ti:Fe2O3) photoanodes are investigated to this aim, and their performance is tuned by engineering the semiconductor surface with a thin layer of Co-based cocatalysts, i.e., cobalt iron oxide (CoFeO x ) and cobalt phosphate (CoPi). Interestingly, the electrode modified with CoPi shows improved efficiency and selectivity toward the final product FDCA The source of this enhancement is correlated to the effect of the cocatalyst on the charge carrier dynamics, which is investigated by electrochemical impedance spectroscopy and intensity-modulated photocurrent spectroscopy analysis. In addition, the results of the latter are interpreted through a novel approach called Lasso distribution of relaxation time, revealing that CoPi cocatalyst is effective in the suppression of the recombination processes and in the enhancement of direct hole transfer to TEMPO.
2022
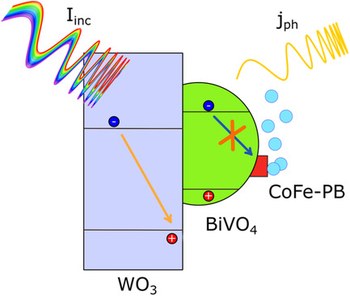
Charge Separation Efficiency in WO3/BiVO4 Photoanodes with CoFe Prussian Blue Catalyst Studied by Wavelength-Dependent Intensity-Modulated Photocurrent Spectroscopy
Sol. RRL, 2022, 6: 2200108
The understanding of charge carrier dynamics in complex heterojunctions is of the utmost importance for the performance optimization of photoelectrochemical cells, especially in operando. Intensity-modulated photocurrent spectroscopy (IMPS) is a powerful tool to this aim, but the information content provided by this technique can be further enhanced by selectively probing each layer of complex heterojunctions by means of multiple excitation sources. Herein, the charge carrier dynamics of a WO3/BiVO4/CoFe–PB heterojunction, used in a conventional three electrode cell for water splitting, is studied using wavelength-dependent IMPS (WD-IMPS). The proposed data analysis allows us to identify the occurrence of interface recombination processes affecting the semiconductor junction, as well as the positive contribution of the inorganic complex catalyst on the charge separation efficiency of the BiVO4 layer. The deep understanding of the fate of charge carriers in the studied photoanode validates WD-IMPS as a straightforward method to widen the understanding of such structures.
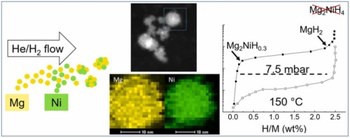
Structure and hydrogen sorption properties of Mg-Mg2Ni nanoparticles prepared by gas phase condensation
Journal of Alloys and Compounds, Volume 911, 2022, 165014
The aim of this work is to investigate the hydrogen sorption kinetics and thermodynamics of Mg-Ni nanoparticles at relatively low temperature in relation to their microstructure. To this purpose, Mg-Ni nanoparticles (20 at% Ni) were prepared by gas phase condensation employing two thermal vapour sources. In the as-prepared state, Mg and Ni are mixed within individual nanoparticles, but the intermetallic Mg2Ni compound is not fully formed. After keeping the nanoparticles at 150 °C for two hours under high vacuum or at a mild hydrogen pressure of 0.15 bar, the formation of a Mg-Mg2Ni or MgH2-Mg2NiH0.3 nanocomposite is observed. Subsequently, fast kinetics of hydrogen sorption are recorded at 150 °C with activation energy of 80±8 kJ/mol (absorption) and 60±6 kJ/mol (desorption). However, the maximum hydrogen storage capacity is limited to 2.5 wt% because the transformation from Mg2NiH0.3 to Mg2NiH4 does not take place at 150 °C even at pressures well above the expected thermodynamic equilibrium. Therefore, only the transformation Mg↔MgH2 contributes to the reversible storage capacity. The corresponding equilibrium pressure determined by pressure-composition isotherms of absorption and desorption at 150 °C is 7.5 mbar, very close to the extrapolated value for bulk Mg. The partial replacement of Ni with Fe does not significantly alter the thermodynamics and kinetics of hydrogen sorption. The structure and hydrogen sorption properties of Mg-Ni nanoparticles are compared to those of Mg-Ti nanoparticles prepared by a similar procedure.