2019
Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole–Quinoline Atropisomers
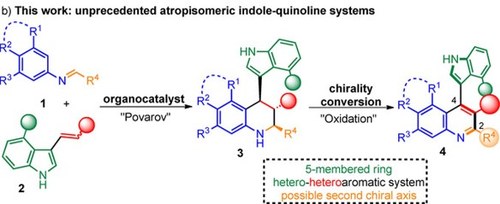
Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole–Quinoline Atropisomers
G. D. Bisag, D.Pecorari, A. Mazzanti, L. Bernardi, M. Fochi, G. Bencivenni, G. Bertuzzi, and V. Corti
Chem. Eur. J. 2019, 25, 15694 – 15701
Axial Chirality at the Boron−Carbon Bond: Synthesis, Stereodynamic Analysis, and Atropisomeric Resolution of 6‑Aryl-5,6- dihydrodibenzo[c,e][1,2]azaborinines

Axial Chirality at the Boron−Carbon Bond: Synthesis, Stereodynamic Analysis, and Atropisomeric Resolution of 6‑Aryl-5,6- dihydrodibenzo[c,e][1,2]azaborinines
A. Mazzanti, M. Boffa, E. Marotta, M. Mancinelli
J. Org. Chem. 2019, 84, 12253−12258
https://doi.org/10.1021/acs.joc.9b01550
Cover: Τhe background of the cover shows the Romanesque Basilica of San Luca, which looks after the City of Bologna and its University. The form resembles the activation energy between two atropisomers. The dynamic properties of the boron–carbon stereogenic axis have been investigated in detail by different techniques such as dynamic NMR, dynamic HPLC, and racemization kinetics of atropisomers, yielding steric rotational barriers much lower than that of the "standard" carbon–carbon stereogenic axes. Absolute configuration was assigned with ECD to stable atropisomers.
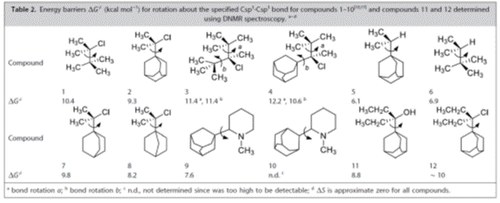
Rotation Barriers of 1‐Adamantyl‐Csp3 Bonds Measured with Dynamic NMR.
Enantioselective Desymmetrization of 1,4-Dihydropyridines by Oxidative NHC Catalysis.
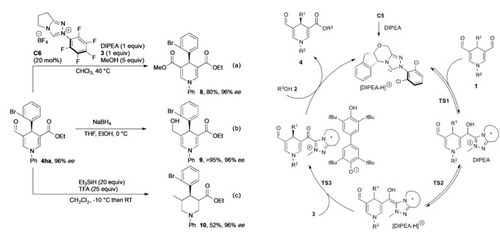
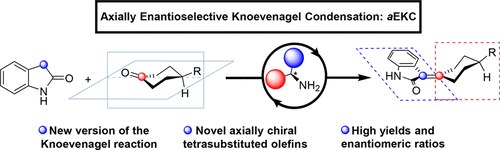
Direct Access to Alkylideneoxindoles via Axially Enantioselective Knoevenagel Condensation
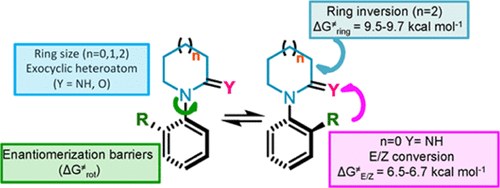
Conformational and Stereodynamic Behavior of Five- to Seven-Membered 1-Aryl-2-iminoazacycloalkanes.
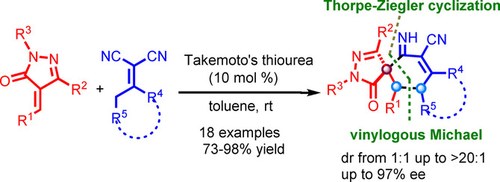
Asymmetric Synthesis of Pyrazolone Fused Spirocyclohexeneimines via a Vinylogous Michael/Cyclization Cascade Reaction
Deuterium Incorporation Protects Cells from Oxidative Damage
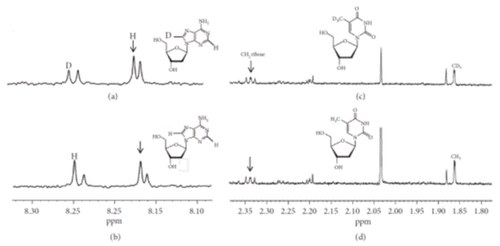
Deuterium Incorporation Protects Cells from Oxidative Damage
P. Sestili, M. Brigotti, C. Calcabrini, E. Turrini, V. Arfilli, D. Carnicelli, M. Lucarini, A. Mazzanti, A. Milelli, V. Righi, C. Fimognari
Oxidative Medicine and Cellular Longevity 2019, 1-13
Predictive chirality sensing via Schiff base formation.
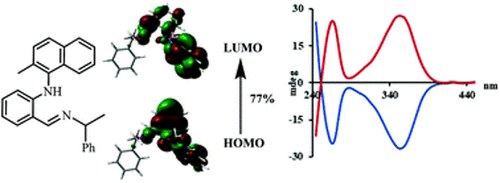
Predictive chirality sensing via Schiff base formation
S. L. Pilicer, M. Mancinelli, A. Mazzanti and C. Wolf
Org. Biomol. Chem., 2019, 17, 6699
Determination of the absolute configuration of conformationally flexible molecules by simulation of chiro-optical spectra: a case study.
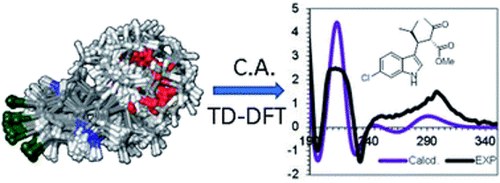
Determination of the absolute configuration of conformationally flexible molecules by simulation of chiro-optical spectra: a case study
Michele Mancinelli, Roberta Franzini, Andrea Renzetti, Emanuela Marotta, Claudio Villani and Andrea Mazzanti
RSC Adv., 2019, 9, 18165–18175
Nitrobutadienes as powerful benzannulating agents: Anunprecedented easy access to rare nitroindoles

Nitrobutadienes as powerful benzannulating agents: Anunprecedented easy access to rare nitroindoles
A. Pagano, M. Mancinelli, L. Bianchi, G. Giorgi, M. Maccagno, G. Petrillo, C. Tavani
Tetrahedron 2019, 75, 4506-4515
Metal and Oxidant-Free Brønsted Acid-Mediated Cascade Reaction to Substituted Benzofurans
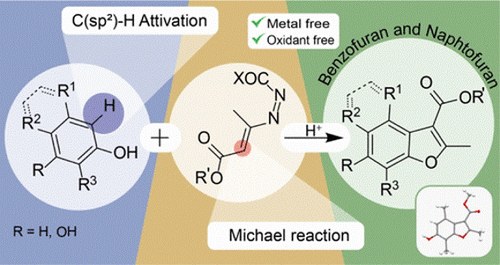
Metal and Oxidant-Free Brønsted Acid-Mediated Cascade Reaction to Substituted Benzofurans
G. Mari, C. Ciccolini, L. De Crescentini, G. Favi, S. Santeusanio, M. Mancinelli, F. Mantellini
J. Org. Chem. 2019, 84, 10814−10824
Adsorption of a Chiral Amine on Alginate Gel Beads and Evaluation of its Efficiency as Heterogeneous Enantioselective Catalyst
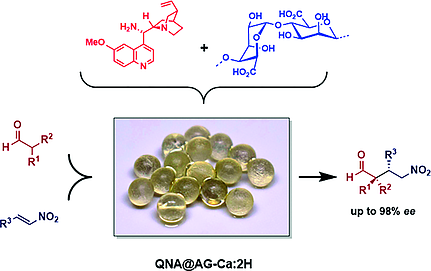
Adsorption of a Chiral Amine on Alginate Gel Beads and Evaluation of its Efficiency as Heterogeneous Enantioselective Catalyst
Daniel Antonio Aguilera, Lisa Spinozzi Di Sante, Asja Pettignano, Riccardo Riccioli, Joël Roeske, Luce Albergati, Vasco Corti, Mariafrancesca Fochi, Luca Bernardi, Françoise Quignard, and Nathalie Tanchoux
Eur. J. Org. Chem. 2019, 3842–3849
Organocatalytic Desymmetrization Reactions for the Synthesis of Axially Chiral Compounds
Organocatalytic Desymmetrization Reactions for the Synthesis of Axially Chiral Compounds
Nicola Di Iorio, Simone Crotti, Giorgio Bencivenni*
Chem. Rec. 2019, 19, 2095–2104