2015
A chelating diisocyanide ligand for cyclometalated Ir(III) complexes with strong and tunable luminescence
A chelating diisocyanide ligand for cyclometalated Ir(III) complexes with strong and tunable luminescence
F. Monti, A. Baschieri, E. Matteucci, A. Mazzanti, L. Sambri, A. Barbieria, N. Armaroli
Faraday Discuss., 2015, 185, 233-248
An Unexpected Pathway to Enantiomerization of Hemithioketals in TolueneInvolving a Dimeric Transition State: A Combined Experimental andComputational Study
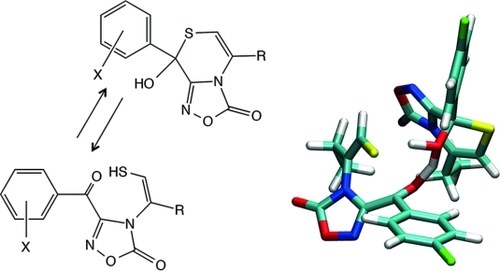
An Unexpected Pathway to Enantiomerization of Hemithioketals in TolueneInvolving a Dimeric Transition State: A Combined Experimental andComputational Study
A. Bottoni, M. Calvaresi, B. Cosimelli, A. Mazzanti, M. Rambaldi, D. Spinelli
Eur. J. Org. Chem. 2015, 4353–4357
APTES mediated modular modification of regenerated silk fibroin in a water solution
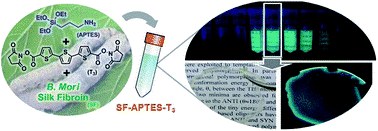
APTES mediated modular modification of regenerated silk fibroin in a water solution
A. Sagnella, M. Zambianchi, M. Durso, T. Posati, A. Del Rio, A. Donnadio, A. Mazzanti, A. Pistone, G. Ruani, R. Zamboni, V. Benfenati, M. Melucci
RSC Adv., 2015, 5, 63401-53406
Catalytic Asymmetric Addition of Meldrum’sAcid, Malononitrile,and 1,3-Dicarbonyls to ortho-Quinone Methides Generated In SituUnder Basic Conditions
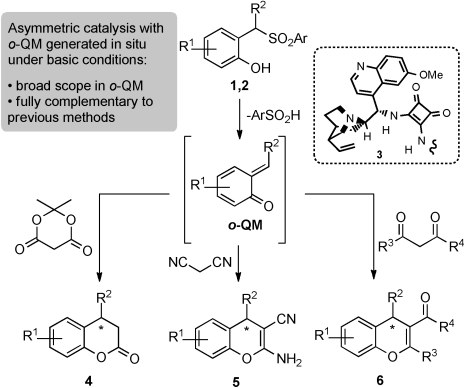
Catalytic Asymmetric Addition of Meldrum’sAcid, Malononitrile,and 1,3-Dicarbonyls to ortho-Quinone Methides Generated In SituUnder Basic Conditions
L. Caruana, M. Mondatori, V. Corti, S. Morales, A. Mazzanti, M. Fochi, L. Bernardi
Chem. Eur.J. 2015, 21, 6037 –6041
Catalytic Asymmetric Reactions of 4-Substituted IndoleswithNitroethene:ADirect EntrytoErgot Alkaloid Structures
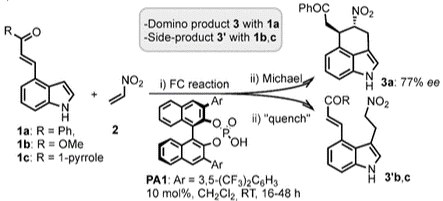
Catalytic Asymmetric Reactions of 4-Substituted Indoles with Nitroethene: A Direct Entry to Ergot Alkaloid Structures
S. Romanini, E. Galletti, L. Caruana, A. Mazzanti, F. Himo, S. Santoro, M. Fochi, L. Bernardi
Chem. Eur. J. 2015, 21, 17578 –17582
Catalytic highly enantioselective transfer hydrogenation of b-trifluoromethyl nitroalkenes. An easy and general entry to optically active b-trifluoromethyl amines
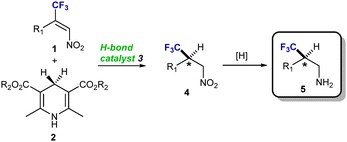
Catalytic highly enantioselective transfer hydrogenation of b-trifluoromethyl nitroalkenes. An easy and general entry to optically active b-trifluoromethyl amines
E. Martinelli, A. C. Vicini, M. Mancinelli, A. Mazzanti, P. Zani, L. Bernardi, M. Fochi
Chem. Commun., 2015, 51, 658--660
Chiral nanostructuring of multivalent macrocycles in solution and on surfaces
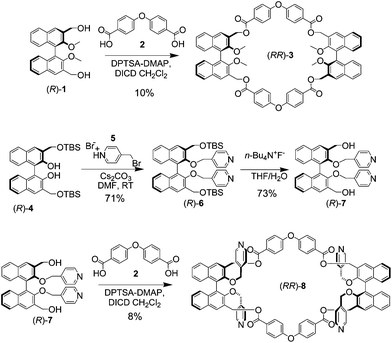
Chiral nanostructuring of multivalent macrocycles in solution and on surfaces
M. Caricato, A. Delforge, D. Bonifazi, D. Dondi, A. Mazzanti, D. Pasini
Org. Biomol. Chem., 2015, 13, 3593–3601
Enantioselective Preparation, Conformational Analysis and Absolute Configuration of Highly Substituted Aziridines
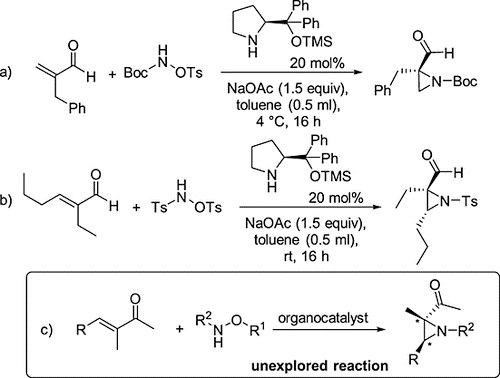
Enantioselective Preparation, Conformational Analysis and Absolute Configuration of Highly Substituted Aziridines
G. Bencivenni, P. Righi, L. Lunazzi, S. Ranieri, M. Mancinelli, A. Mazzanti
Chirality 2015, 27, 875–887
Long-Range Bonding/Nonbonding Interactions: A Donor–Acceptor Resonance Studied by Dynamic NMR
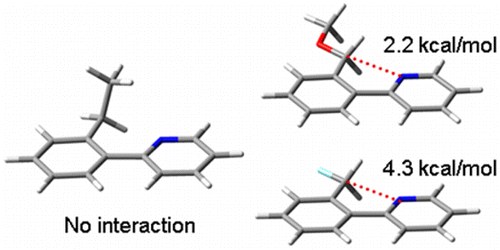
Long-Range Bonding/Nonbonding Interactions: A Donor–Acceptor Resonance Studied by Dynamic NMR
R. Ruzziconi, S. Lepri, F. Buonerba, M. Schlosser, M. Mancinelli, S. Ranieri, L. Prati, A. Mazzanti
Org. Lett. 2015, 17, 2740−2743
https://doi.org/10.1021/acs.orglett.5b01152
Me2Zn-Mediated Catalytic Enantio- and Diastereoselective Addition of TosMIC to Ketones
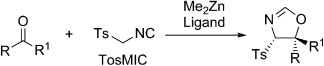
Me2Zn-Mediated Catalytic Enantio- and Diastereoselective Addition of TosMIC to Ketones
A. R. Keeri, A. Gualandi, A. Mazzanti, J. Lewinski, P. G. Cozzi
Chem. Eur. J. 2015, 21,18949 –18952
Organocatalytic Atroposelective Formal Diels−Alder Desymmetrization of N‑Arylmaleimides
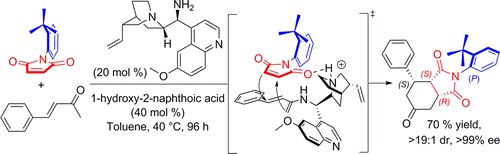
Organocatalytic Atroposelective Formal Diels−Alder Desymmetrization of N‑Arylmaleimides
F. Eudier, P. Righi, A. Mazzanti, A. Ciogli, G. Bencivenni
Org. Lett. 2015, 17, 1728−1731
Structure and conformational dynamics of an aromatic sulfonamide: NMR, X-Ray and computational studies
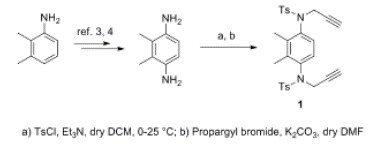
Structure and conformational dynamics of an aromatic sulfonamide: NMR, X-Ray and computational studies
S. Menichetti, C. Biagioli, C. Viglianisi, L. Tofani, L. Lunazzi, M. Mancinelli, A. Mazzanti
Arkivic 2015, iv, 66-79
Synthesis and antimicrobial activity of novel structural hybrids ofbenzofuroxan and benzothiazole derivatives
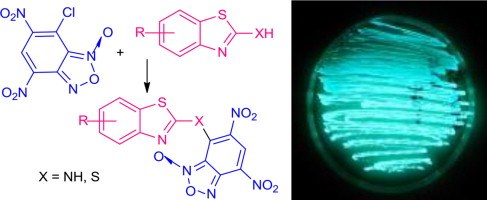
Synthesis and antimicrobial activity of novel structural hybrids ofbenzofuroxan and benzothiazole derivatives
E. Chugunova, C. Boga, I. Sazykin, S. Cino, G. Micheletti, A. Mazzanti, M. Sazykina, A. Burilov, L. Khmelevtsova, N. Kostina
European Journal of Medicinal Chemistry 2015, 93, 349-359
Vinylogous Reactivity of Oxindoles Bearing Nonsymmetric 3‑Alkylidene Groups
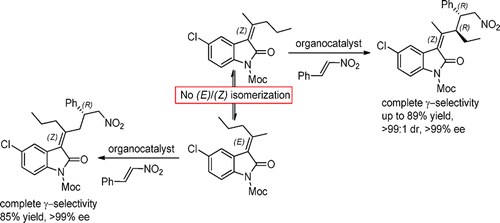
Vinylogous Reactivity of Oxindoles Bearing Nonsymmetric 3‑Alkylidene Groups
N. Di Iorio, P. Righi, S. Ranieri, A. Mazzanti, R. G. Margutta, G. Bencivenni
J. Org. Chem. 2015, 80, 7158−7171
Alginic acid aerogel: a heterogeneous Brønsted acid promoter for the direct Mannich reaction.
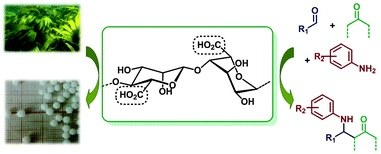
Alginic acid aerogel: a heterogeneous Brønsted acid promoter for the direct Mannich reaction.
Asja Pettignano, Luca Bernardi, Mariafrancesca Fochi, Lorenzo Geraci, Mike Robitzer, Nathalie Tanchoux and Françoise Quignard
New J. Chem., 2015, 39, 4222-4226
https://pubs.rsc.org/en/content/articlelanding/2015/NJ/c5nj00349k#!divAbstract
The Emergence of Quinone Methides in Asymmetric Organocatalysis
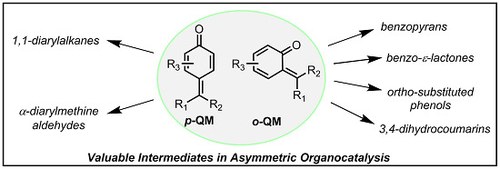
The Emergence of Quinone Methides in Asymmetric Organocatalysis
Mariafrancesca Fochi, Lorenzo Caruana and Luca Bernardi
Molecules 2015, 20, 11733-11764
OPEN ACCESS
https://www.mdpi.com/1420-3049/20/7/11733
Organocatalytic Asymmetric Conjugate Additions to Cyclopent-1-enecarbaldehyde: A Critical Assessment of Organocatalytic Approaches towards the Telaprevir Bicyclic Core
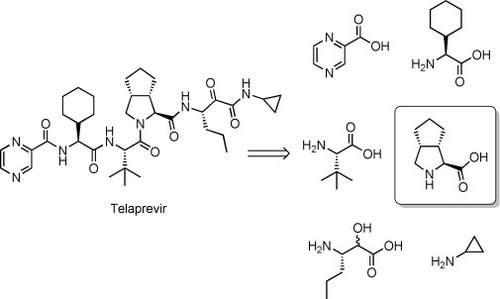
Organocatalytic Asymmetric Conjugate Additions to Cyclopent-1-enecarbaldehyde: A Critical Assessment of Organocatalytic Approaches towards the Telaprevir Bicyclic Core
Luca Bernardi, Mariafrancesca Fochi, Riccardo Carbone, Ada Martinelli, Martin E. Fox, Christopher J. Cobley, Bhaskar Kandagatla, Srinivas Oruganti, Vilas H. Dahanukar and Armando Carlone
Chem. Eur. J. 2015, 21, 19208–19222