Asymmetric Synthesis Lab
We enjoy playing with Asymmetric Synthesis. Our ambition is to control the spatial arrangement of stereogenic centers and axes using organo- and metal catalyzed reactions. Our research fields are oriented to the study of asymmetric vinylogous reactions and the development of processes which enables the enantioselective synthesis of atropisomers.
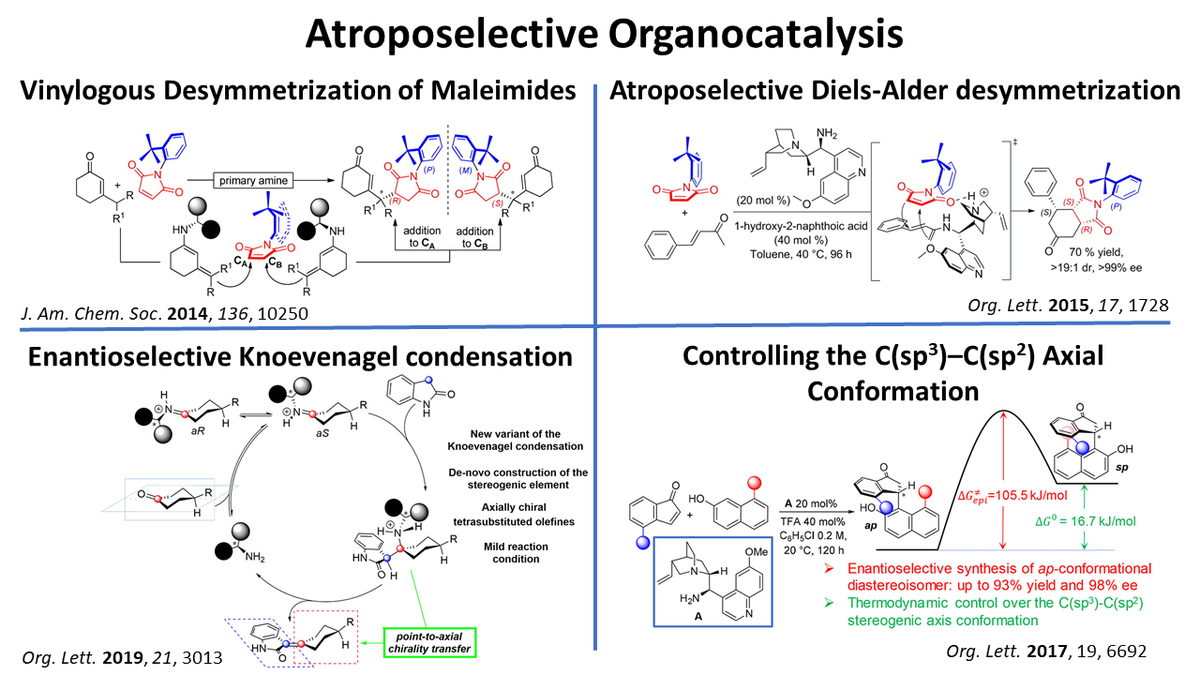
Organocatalytic enantioselective formation of atropisomers:
-
Enantioselective organocatalysis has been successfully applied to the synthesis of atropisomers
-
Desymmetrization of N-arylmaleimmides by nucleophilic attack on their prochiral double bond afforded atropisomeric succinimides
-
Axially chiral cyclohexylidene oxindoles were selectively obtained by means of organocatalytic Knoevenagel condensation. Insights on the mechanism were obtained by DFT methods.
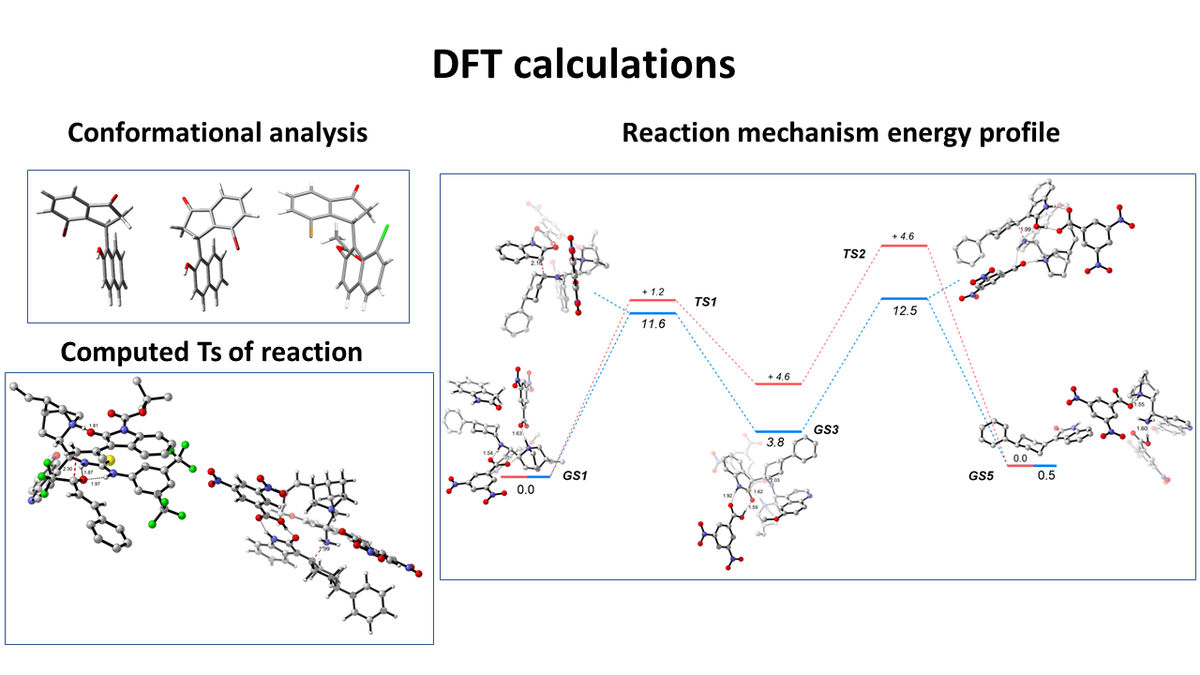
DFT calculations
- Computational studies for the comprehension of the reaction mechanisms
- Catalyst design
- Conformational analysis
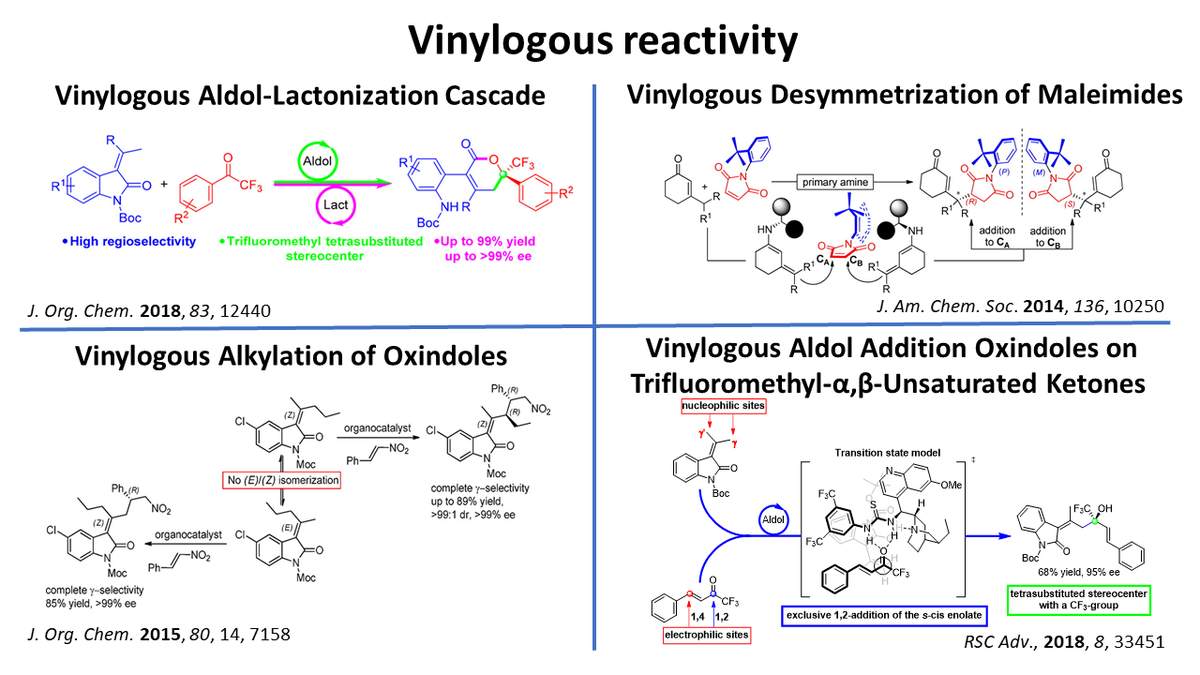
New organocatalytic enantioselective vinylogous reactions:
-
Vinylogous reactivity is a valuable strategy for the remote modification of a molecule.
-
Vinylogous addition of alkylidene oxindole on aryl trifluoromethyl ketone resulted in a rare aldol reaction-lactonization cascade. The reaction, catalyzed by a bifunctional tertiary amine, provides an efficient entry to enantioenriched trifluoromethylated α,β-unsaturated δ-lactones.
-
The addition on α,β-unsaturated trifluoromethyl ketones provided an efficient preparation of enantioenriched trifluoromethylated allylic alcohols
CONTACTS
Prof. Dr. Giorgio Bencivenni, PhD
Prof. of Organic Chemistry.
Teaching: Chimica Organica Superiore, Chimica Organica 2, Chimica Organica 1 con Laboratorio
viale del Risorgimento 4, 40136, Bologna-Italy
+390512093624
http://www.studentidisabili.unibo.it/chi-siamo/contatti
https://twitter.com/gbbencio; https://twitter.com/LabSynthesis
Prof. Dr. Paolo Righi, PhD
Prof. of Organic Chemistry
Teaching: Chimica Organica 2, Chimica Organica Magistrale
viale del Risorgimento 4, 40136, Bologna-Italy
+39 051 20 9 3640
Prof. Emanuela Marotta
Prof. of Organic Chemistry
Teaching: Chimica Organica 2
viale del Risorgimento 4, 40136, Bologna-Italy
+39 051 20 9 3640
Recent Publications
-
Direct Access to Alkylideneoxindoles via Axially Enantioselective Knoevenagel Condensation. Org. Lett. 2019, 21, 9, 3013–3017
The organocatalytic axially enantioselective Knoevenagel condensation between prochiral cyclohexanones and oxindoles is presented. The reaction, promoted by a primary amine, proceeded smoothly and furnished unprecedented examples of novel cyclohexylidene oxindoles displaying axial chirality.
-
Central‐to‐Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole–Quinoline Atropisomers
The first stereoselective synthesis of enantioenriched axially chiral indole–quinoline systems is presented. The strategy takes advantage of an organocatalytic enantioselective Povarov cycloaddition of 3‐alkenylindoles and N‐arylimines, followed by an oxidative central‐to‐axial chirality conversion process, allowing for access to previously unreported axially chiral indole–quinoline biaryls. The methodology is also implemented for the design and the preparation of challenging compounds exhibiting two stereogenic axes. DFT calculations shed light on the stereoselectivity of the central‐to‐axial chirality conversion, showing unconventional behavior
-
Organocatalytic Desymmetrization Reactions for the Synthesis of Axially Chiral Compounds. Chem. Rec. 2019, 19, 2095–2104
The enantioselective synthesis of atropisomers is an emerging field, that in recent years reached fundamental results and put the bases for innovative applications. Organocatalysis is playing a central role in the realization of original synthesis for novel atropisomeric scaffolds. In this short review, we would like to highlight the results obtained by our group and others in the field of axially enantioselective desymmetrization reactions using organocatalysis as main strategy
-
Enantioselective Synthesis of Trifluoromethyl α,β-Unsaturated δ-Lactones via Vinylogous Aldol-Lactonization Cascade. J. Org. Chem. 2018, 83, 20, 12440–12448
The novel vinylogous aldol-lactonization cascade of alkylidene oxindole with trifluoromethyl ketones is presented. The reaction, catalyzed by a bifunctional tertiary amine, provides an efficient application of the vinylogous reactivity of alkylidene oxindoles for the preparation of enantioenriched trifluoromethylated α,β-unsaturated δ-lactones.
-
Controlling the C(sp3)–C(sp2) Axial Conformation in the Enantioselective Friedel–Crafts-Type Alkylation of β-Naphthols with Inden-1-ones. Org. Lett. 2017, 19, 24, 6692–6695
The Friedel–Crafts-type reaction between properly functionalized inden-1-ones and 2-naphthols generates a hindered single bond which displays a unique preference for an antiperiplanar conformational diastereoisomer. The steric hindrance and the presence of an enantioenriched stereogenic center control the distribution of the two diastereomeric conformers at equilibrium and increase the energy for the rotation of the C(sp3)–C(sp2) single bond.
-
Vinylogous Reactivity of Oxindoles Bearing Nonsymmetric 3-Alkylidene Groups. J. Org. Chem. 2015, 80, 14, 7158–7171
The γ-functionalization of oxindoles bearing nonsymmetric 3-alkylidene groups via vinylogous Michael-type addition to nitroolefins was realized. The suppression of the interconversion between the E and Z isomers of the starting oxindoles allowed a site-specific diastereoselective and enantioselective transformation. Specific experiments allowed us to establish the rate-determining step of the reaction and to advance a robust hypothesis for the exclusive formation of an s-cis enolate as the only reactive intermediate.
-
Organocatalytic Atroposelective Formal Diels–Alder Desymmetrization of N-Arylmaleimides. Org. Lett. 2015, 17, 7, 1728–1731
The atroposelective desymmetrization of N-arylmaleimides was realized by means of a primary amine catalyzed Diels–Alder reaction of enones. The chiral axis as new element of chirality is generated under the remote control of the catalyst that selectively drives the formal Diels–Alder reaction through an exclusive stereochemical outcome.
-
Remote Control of Axial Chirality: Aminocatalytic Desymmetrization of N-Arylmaleimides via Vinylogous Michael Addition. J. Am. Chem. Soc. 2014, 136, 29, 10250–10253
Remote control of the axial chirality of N-(2-t-butylphenyl)succinimides was realized via the vinylogous Michael addition of 3-substituted cyclohexenones to N-(2-t-butylphenyl)maleimides. 9-Amino(9-deoxy)epi-quinine promoted the enantioselective desymmetrization, leading to atropisomeric succinimides with two adjacent stereocenters.