2018
Stereodynamic Analysis of New Atropisomeric 4,7-Di(naphthalen-1-yl)-5,6-dinitro-1 H -indoles
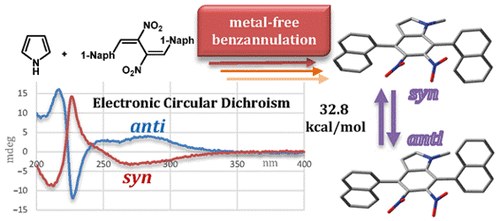
Stereodynamic Analysis of New Atropisomeric 4,7-Di(naphthalen-1-yl)-5,6-dinitro-1 H -indoles
A. Pagano, E. Marotta, A. Mazzanti, G. Petrillo, C. Tavani, M. Mancinelli
Synlett 2018, 29, 2161-2166
https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0037-1609908
Enantioselective Synthesis of Trifluoromethyl α, β-Unsaturated δ-Lactones via Vinylogous Aldol-Lactonization Cascade
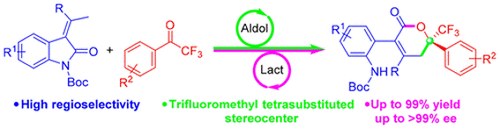
Enantioselective Synthesis of Trifluoromethyl α, β-Unsaturated δ-Lactones via Vinylogous Aldol-Lactonization Cascade
S. Crotti, N. Di Iorio, A. Mazzanti, P. Righi, G. Bencivenni
J. Org. Chem. 2018, 83, 12440-12448
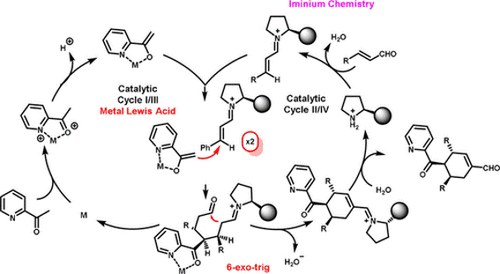
Synergistic Catalysis: Highly Enantioselective Acetyl Aza‐arene Addition to Enals
Quinone‐Fused Pyrazoles through 1, 3‐Dipolar Cycloadditions: Synthesis of Tricyclic Scaffolds and in vitro Cytotoxic Activity Evaluation on Glioblastoma Cancer Cells
Quinone‐Fused Pyrazoles through 1, 3‐Dipolar Cycloadditions: Synthesis of Tricyclic Scaffolds and in vitro Cytotoxic Activity Evaluation on Glioblastoma Cancer Cells
G. Bertuzzi, S. Crotti, P. Calandro, B. F. Bonini, I. Monaco, E. Locatelli, M. Fochi, P. Zani, E. Strocchi, A. Mazzanti, M. Chiariello, M. Comes Franchini
ChemMedChem 2018, 13, 1744-1750
Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones
Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones
M. Meazza, M. Kamlar, L. Jašíková, B. Formánek, A. Mazzanti, J. Roithová, J. Veselý, R. Rios
Chemical Science 2018, 9, 6368-6373
https://doi.org/10.1039/C8SC00913A
Click to view Correction:
Catalytic Enantioselective Povarov Reactions of Ferrocenecarbaldehyde‐Derived Imines–Brønsted Acid Catalysis at Parts‐Per‐Million Level Loading
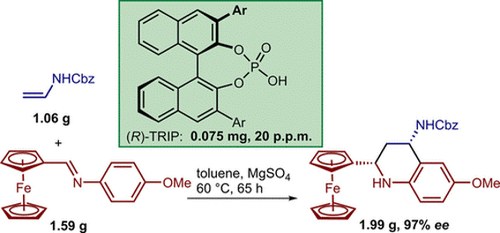
Catalytic Enantioselective Povarov Reactions of Ferrocenecarbaldehyde‐Derived Imines–Brønsted Acid Catalysis at Parts‐Per‐Million Level Loading
Dragana Stevanović, Giulio Bertuzzi, Andrea Mazzanti, Mariafrancesca Fochi and Luca Bernardi
Advanced Synthesis & Catalysis 2018, 360, 893-900
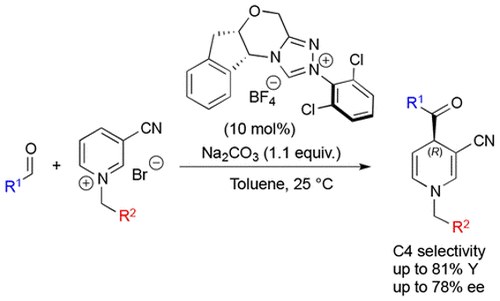
Enantioselective Dearomatization of Alkylpyridiniums by N-Heterocyclic Carbene-Catalyzed Nucleophilic Acylation
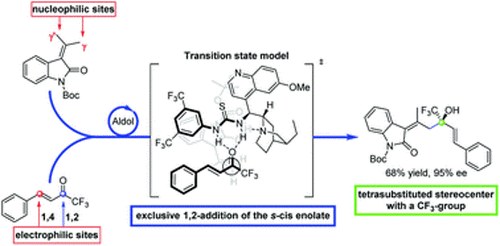
Asymmetric vinylogous aldol addition of alkylidene oxindoles on trifluoromethyl-α, β-unsaturated ketones
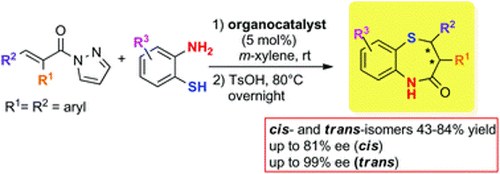
Catalytic enantioselective one-pot approach to cis-and trans-2, 3-diaryl substituted 1, 5-benzothiazepines
An organocatalytic enantioselective direct α-heteroarylation of aldehydes with isoquinoline N-oxides
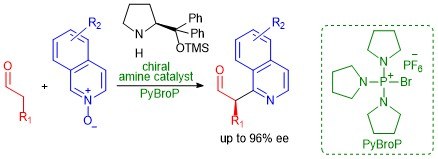
An organocatalytic enantioselective direct α-heteroarylation of aldehydes with isoquinoline N-oxides
Giulio Bertuzzi, Daniel Pecorari, Luca Bernardi, and Mariafrancesca Fochi
Chem. Commun., 2018, 54, 3977–3980
https://pubs.rsc.org/en/content/articlelanding/2018/cc/c8cc01735b#!divAbstract
gamma-Regioselective Functionalization of 3-Alkenylindoles via 1,6-Addition to Extended Alkylideneindolenine Intermediates

gamma-Regioselective Functionalization of 3-Alkenylindoles via 1,6-Addition to Extended Alkylideneindolenine Intermediates
Giulio Bertuzzi, Lucia Lenti, Denisa Giorgiana Bisag, Mariafrancesca Fochi, Marino Petrini, and Luca Bernardi
Adv. Synth. Catal. 2018, 360, 1296 – 1302
Nucleophilic Dearomatization of Activated Pyridines
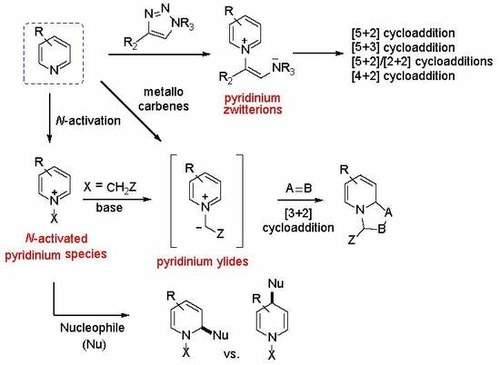
Nucleophilic Dearomatization of Activated Pyridines
Giulio Bertuzzi, Luca Bernardi, and Mariafrancesca Fochi
Catalysts 2018, 8, 632
OPEN ACCESS