2016
A Mesoionic Carbene as Neutral Ligand for Phosphorescent Cationic Ir(III) Complexes
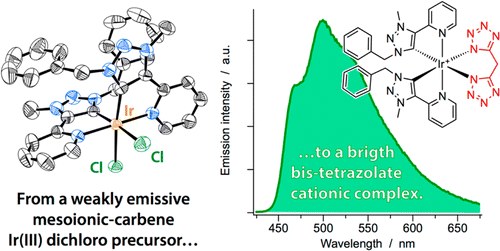
A Mesoionic Carbene as Neutral Ligand for Phosphorescent Cationic Ir(III) Complexes
A. Baschieri, F. Monti, E. Matteucci, A. Mazzanti, A. Barbieri, N. Armaroli, L. Sambri
Inorg. Chem. 2016, 55, 7912−7919
An Atropisomerically Enforced Phosphoric Acid forOrganocatalytic Asymmetric Reactions
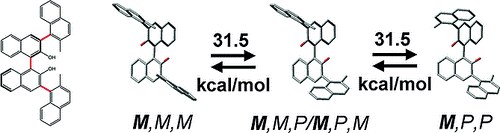
An Atropisomerically Enforced Phosphoric Acid forOrganocatalytic Asymmetric Reactions
L. Bernardi, G. Bolzoni, M. Fochi, M. Mancinelli, A. Mazzanti
Eur. J. Org. Chem. 2016, 3208–3216
Atropisomerism in 3-arylthiazolidine-2-thiones. A combined dynamic NMR and dynamic HPLC study
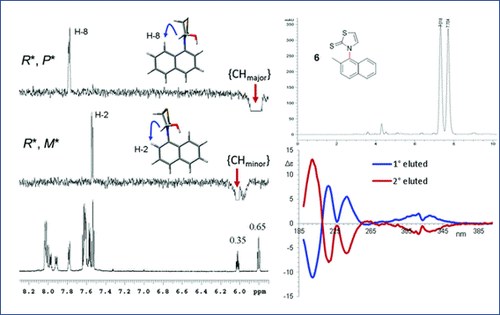
Atropisomerism in 3-arylthiazolidine-2-thiones. A combined dynamic NMR and dynamic HPLC study
A. Ciogli, S. Vivek Kumar, M. Mancinelli, A. Mazzanti, S. Perumal, C. Severi, C. Villani
Org. Biomol. Chem., 2016, 14, 11137
Axial Chirality about Boron–Carbon Bond: Atropisomeric Azaborines
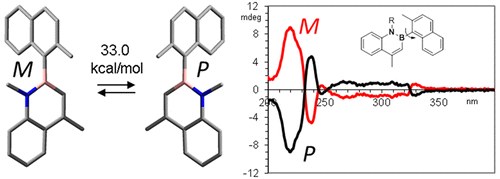
Axial Chirality about Boron–Carbon Bond: Atropisomeric Azaborines
A. Mazzanti, E. Mercanti, M. Mancinelli
Org. Lett. 2016, 18, 2692−2695
Catalytic Enantioselective Addition of Indoles to Activated N‑Benzylpyridinium Salts: Nucleophilic Dearomatization of Pyridines with Unusual C‑4 Regioselectivity
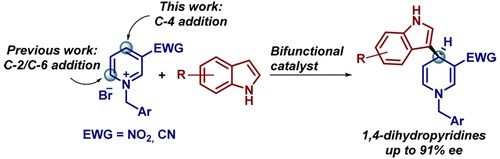
Catalytic Enantioselective Addition of Indoles to Activated N‑Benzylpyridinium Salts: Nucleophilic Dearomatization of Pyridines with Unusual C‑4 Regioselectivity
G. Bertuzzi, A. Sinisi, L. Caruana, A. Mazzanti, M. Fochi, L. Bernardi
ACS Catal. 2016, 6, 6473−6477
Computational and DNMR Analysis of the Conformational Isomers and Stereodynamics of Secondary 2,2′-Bisanilides
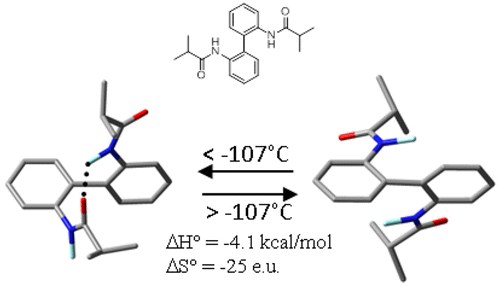
Computational and DNMR Analysis of the Conformational Isomers and Stereodynamics of Secondary 2,2′-Bisanilides
A. Mazzanti, M. Chiarucci, L. Prati, K. W. Bentley, C. Wolf
J. Org. Chem. 2016, 81, 89−99
Enantioselective Organocatalytic Cyclopropanation of Enals Using Benzyl Chlorides
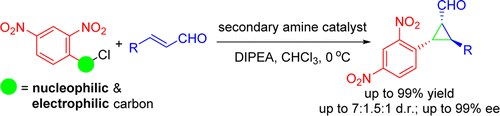
Enantioselective Organocatalytic Cyclopropanation of Enals Using Benzyl Chlorides
M. Meazza, M. Ashe, H. Y. Shin, H. S. Yang, A. Mazzanti, J. W. Yang, R. Rios
J. Org. Chem. 2016, 81, 3488−3500
New azo-decorated N-pyrrolidinylthiazoles: synthesis, properties and an unexpected remote substituent effect transmission
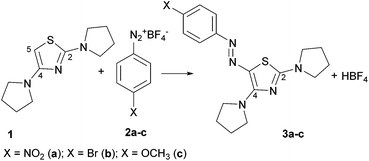
New azo-decorated N-pyrrolidinylthiazoles: synthesis, properties and an unexpected remote substituent effect transmission
C. Boga, S. Cino, G. Micheletti, D. Padovan, L. Prati, A. Mazzanti, N. Zanna
Org. Biomol. Chem., 2016, 14, 7061-7068
Straightforward synthesis of a novel ring-fused pyrazole-lactam andin vitro cytotoxic activity on cancer cell lines
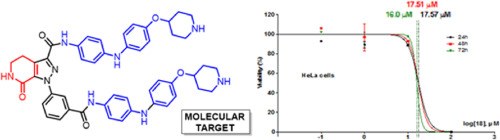
Straightforward synthesis of a novel ring-fused pyrazole-lactam andin vitro cytotoxic activity on cancer cell lines
G. Bertuzzi, E. Locatelli, D. Colecchia, P. Calandro, B.F. Bonini, J.Z. Chandanshive, A. Mazzanti, P. Zani, M. Chiariello, M. Comes Franchini
European Journal of Medicinal Chemistry 2016, 117, 1-7
Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones
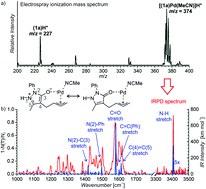
Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones
M. Meazza, M. Kamlar, L. Jašíková, Bedřich Formánek, A. Mazzanti, Jana Roithová, Jan Veselý, R. Rios
Chem. Sci., 2018, 9, 6368-6373
Targeting remote axial chirality control ofN-(2-tert-butylphenyl)succinimides by means of Michael addition type reactions
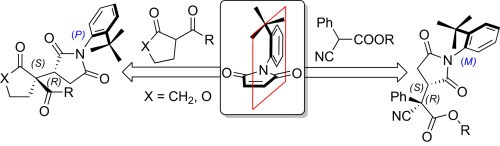
Targeting remote axial chirality control ofN-(2-tert-butylphenyl)succinimides by means of Michael addition type reactions
N. Di Iorio, F. Champavert, A. Erice, P. Righi, A. Mazzanti, G. Bencivenni
Tetrahedron 2016, 72, 5191-5201
Reversible modulation of the activity of thiourea catalysts with anions: a simple approach to switchable asymmetric catalysis
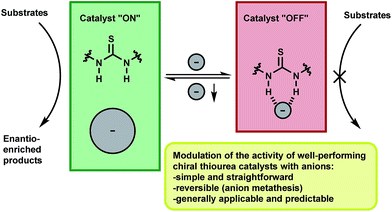
Reversible modulation of the activity of thiourea catalysts with anions: a simple approach to switchable asymmetric catalysis
Giacomo Foli, Cecilia Sasso D’Elia, Mariafrancesca Fochi and Luca Bernardi
RSC Adv. 2016, 6, 66490-66494
https://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra12732k/unauth#!divAbstract
A General Catalytic Enantioselective Transfer Hydrogenation Reaction of β,β-Disubstituted Nitroalkenes Promoted by a Simple Organocatalyst
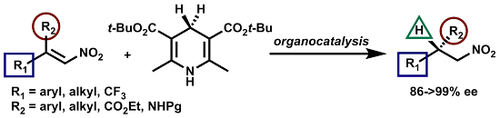
A General Catalytic Enantioselective Transfer Hydrogenation Reaction of β,β-Disubstituted Nitroalkenes Promoted by a Simple Organocatalyst
Luca Bernardi and Mariafrancesca Fochi
Molecules 2016 vol. 21 (8) Article number 1000 pp.1-14
OPEN ACCESS
Organocatalytic Enantioselective Transfer Hydrogenation of -Amino Nitroolefins
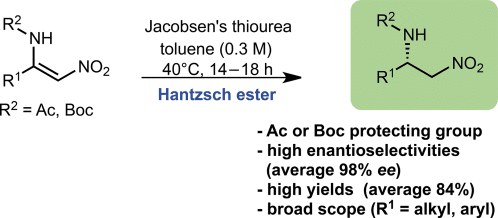
Organocatalytic Enantioselective Transfer Hydrogenation of b-Amino Nitroolefins
Antonino Ferraro, Luca Bernardi and Mariafrancesca Fochi
Adv. Synth. Catal. 2016, 358, 1561 – 1565
Synthesis and Preliminary Results on the Catalytic Activity of Metal Complexes obtained from C2‐Symmetric Ligands Derived from R‐(+)‐Betti base
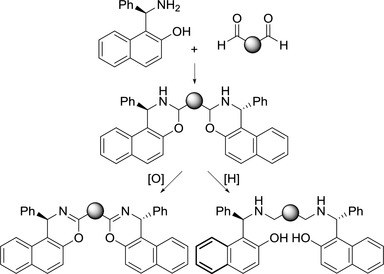
Synthesis and Preliminary Results on the Catalytic Activity of Metal Complexes obtained from C2‐Symmetric Ligands Derived from R‐(+)‐Betti base
T. Rigotti, P. Righi, E. Marotta, C. Paolucci
Chemsitry SELECT 2016, 1, 2624 - 2629