NanoPhage - An Innovative Hybrid Platform for Targeted Sono- Photo- Dynamic Cancer Therapy My First AIRC Grant (MFAG)
Background
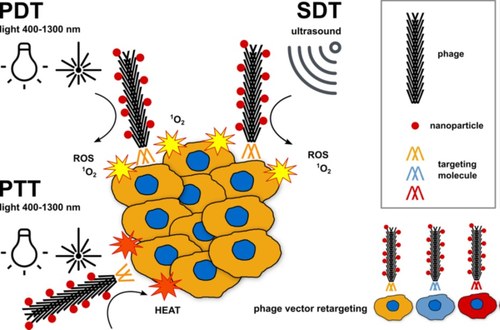
Fullerenes and gold nanoparticles (AuNPs) are under extensive investigation because of their potential for tumor imaging and therapy [agents for photodynamic (PDT), sonodynamic (SDT) and photothermal (PTT) therapy]. Despite the improvements that fullerenes and AuNPs display with respect to current cancer therapy and imaging techniques, they still present important restrictions in their use due to 1)poor water solubility and low biocompatibility; 2)non-specificity of the cellular uptake.
Hypothesis
Phages are naturally occurring viruses that develop high selectivity for certain bacteria, while they are inactive against eukaryotic cells. The great ease of manipulation of the phage genome, allows to display peptides/antibodies on the phage surface, retargeting them to any type of cell. At the same time hundreds of therapeutic and imaging agents can be conjugated onto the phage capsid, providing a robust and flexible platform for anticancer approaches.
Aims
NanoPhage will functionalize the phage capsid with fullerenes and AuNPs and target this suicide vector selectively to cancer cells via phage display of single-chain antibodies or tumor-binding peptides. NanoPhage will target HER2 and EGFR receptors, because they are frequently overexpressed in various cancers. An innovative platform for receptor-targeted PDT, SDT and PTT will be developed.
Expected Results
The conjugation of fullerene and AuNPs to receptor-targeted phages will offer the opportunity to create an innovative vector able to exploit the synergistic action of sono- photo- dynamic therapy, that in hypoxic conditions can activate an alternative mechanism of cell killing: thermal ablation (synergistic vector).
Impact On Cancer
NanoPhage will develop a phage-based theranostic platform for targeted cancer treatment and imaging. Nanophage will combine the advantages of immunoconjugates anticancer therapies (specific, highly effective, minimally toxic) with the possibility to load orthogonally hundreds of effectors in the phage platform. Soft and penetrating irradiation sources (ultrasounds and NIR light) will be used to activate the NanoPhages. A double-targeting therapy will be developed, exploiting the selectivity of the NanoPhage and the possibility to have a focused irradiation at the desired site of action, lowering the collateral damage to healthy tissues. Effective targeting of disseminated tumors in vivo and induction of antitumor immunity by NanoPhage treatment will be investigated

Principal Investigator
Matteo Calvaresi
Project partners
Pier Luigi Lollini, Maria Pia Foschini
Team members
Alberto Danielli, Eleonora Turrini, Roberto Saporetti, Luca Ulfo
Collaborators
Annapaola Petrosino, Paolo Emidio Costantini, Matteo Di Giosia, Andrea Cantelli