BOBCAT: BIOCOMPATIBLE BORON CARRIERS FOR BORON NEUTRON CAPTURE THERAPY
Principal Investigator: Dr Tainah D. Marforio
Background
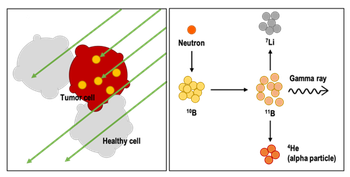
Boron Neutron Capture Therapy (BNCT) is a non-invasive therapeutic modality for locally treating invasive malignant tumors. The effectiveness of the treatment is based on the sufficient and selective accumulation of boron atoms in the tumor tissue, that upon neutron radiation undergo capture reaction, causing the cell apoptosis. Selective accumulation of boron in cancer cells is the major problem in drug development for BNCT. A variety of boron clusters (BCs) have been recently obtained. Because of their chemical structure, these BCs promise to be good candidates in BNCT, since each BC comprises from 10 to 20 boron atoms.
Hypothesis
BCs are insoluble in physiological environment. Their hydrophobicity requires the development of delivery systems able to transport them to the tumor cell. Peptides, proteins and antibodies are promising vehicles for BC delivery. The intrinsic characteristic of the carriers, such as the biocompatibility and tumor targeting activity, can be exploited to effectively and selectively deliver BC to the cancer cells.
Aims
BOBCAT aims to design biocompatible carriers for BCs in BNCT, by means of a combined computational-experimental approach. Virtual screening and de novo design methodologies will identify the best protein/peptide candidates to delivery BCs to cancer cells. Physiologically stable BC@protein/BC@peptide adducts will be synthesized. Boron concentrations within cancer cells will be maximized and the tumor cell-killing of BC@protein/BC@peptide adducts will be demonstrated in BNCT.
Expected Results
The in silico approach will allow to screen thousands of protein/peptide candidates in a cheap and safe way, identifying the most promising candidates for BC delivery. The virtual screening results will be validated by the synthesis of a collection of BC@protein/BC@peptide adducts. The kinetic and thermodynamic parameters of binding will be determined. The most promising complexes will be tested on cellular lines, showing the efficacy of the delivery system, the nanosafety of the complexes and their performances in BNCT.
Impact On Cancer
Despite the impressive clinical results, the dependency on nuclear reactors as neutron sources has limited the interest of clinicians to BNCT. The advent of accelerator-based neutron sources (ABNS) will promote BNCT to a "routine" radiation therapy if an effective boron delivery agent for BNCT will be developed. BOBCAT will provide an innovative and biocompatible platform for BNCT, with a strong translation potential. BOBCAT will help the transition of BNCT from an experimental modality to a promising clinical approach.