MimePept
J. Med. Chem. 2018, 61, 5751−5757
Rossella De Marco,†,∥ Andrea Bedini,‡,∥ Santi Spampinato,*,‡ Lorenzo Comellini,† Junwei Zhao,†
Roberto Artali,§ and Luca Gentilucci*,†
†Department of Chemistry “G. Ciamician”, University of Bologna, Via Selmi 2, 40126 Bologna, Italy
‡Department of Pharmacy and Biotechnology, University of Bologna, Via Irnerio 48, 40126 Bologna, Italy
§Scientia Advice, 20832 Desio, Monza and Brianza, Italy
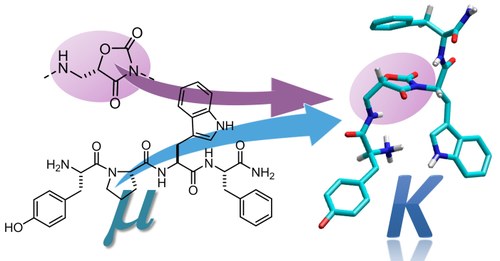
ABSTRACT: Herein we present the expedient synthesis of endomorphin-1 analogues containing stereoisomeric β2-homo-
Freidinger lactam-like scaffolds ([Amo2]EM), and we discuss opioid receptor (OR) affinity, enzymatic stability, functional
activity, in vivo antinociceptive effects, and conformational and molecular docking analysis. Hence, H-Tyr-Amo-Trp-PheNH2
resulted to be a new chemotype of highly stable, selective, partial KOR agonist inducing analgesia, therefore displaying
great potential interest as a painkiller possibly with reduced harmful side effects.
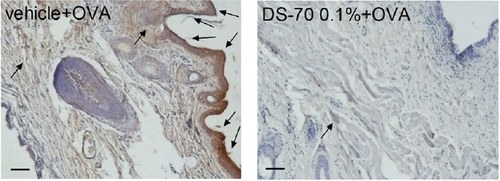
Figure. Topical treatment with DS-70 in guinea pigs challenged with OVA reduces conjunctival levels of α4 integrin. Hot spots of expression in the photomicrographs of the conjunctiva, 24 h after OVA and pretreatment with 0.1% DS-70.
Samantha Deianira Dattoli1,*, Monica Baiula1,*, Rossella De Marco2, Andrea Bedini1, Michele Anselmi2,
Luca Gentilucci2 and Santi Spampinato1
1 Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy, and 2 Department of Chemistry “G. Ciamician”, University of Bologna, Bologna, Italy
BACKGROUND AND PURPOSE. Allergic conjunctivitis is an eye inflammation that involves the infiltration of immune cells into the conjunctiva via cell surface adhesion receptors, such as integrin α4β1. These receptors interact with adhesion molecules expressed on the conjunctival endothelium and may be a target to treat this disease. We synthesized DS-70, a novel α/β-peptidomimetic α4 integrin antagonist, to prevent the conjunctival infiltration of immune cells and clinical symptoms in a model of allergic conjunctivitis.
EXPERIMENTAL APPROACH. In vitro, DS-70 was pharmacologically characterized using a scintillation proximity procedure to measure its affinity for α4β1 integrin, and its effect on cell adhesion mediated by different integrins was also evaluated. The effects of DS-70 on vascular cell adhesion molecule-1 (VCAM-1)-mediated degranulation of a human mast cell line and an eosinophilic cell line, which both express α4β1, and on VCAM-1-mediated phosphorylation of ERK 1/2 in Jurkat E6.1 cells were investigated. Effects of DS-70 administered in the conjunctival fornix of ovalbumin-sensitized guinea pigs were evaluated.
KEY RESULTS. DS-70 bound to integrin α4β1 with nanomolar affinity, prevented the adhesion of α4 integrin-expressing cells, antagonized VCAM-1-mediated degranulation of mast cells and eosinophils and ERK 1/2 phosphorylation. Only 20% was degraded after an 8 h incubation with serum. DS-70 dose-dependently reduced the clinical symptoms of allergic conjunctivitis, conjunctival α4 integrin expression and conjunctival levels of chemokines and cytokines in ovalbumin-sensitized guinea pigs.
CONCLUSIONS AND IMPLICATIONS. These findings highlight the role of α4 integrin in allergic conjunctivitis and suggest that DS-70 is a potential treatment for this condition.
European Journal of Medicinal Chemistry 179 (2019) 527-536
Karol Wtorek a, Roberto Artali c, Justyna Piekielna-Ciesielska a, Jacek Koszuk d, Alicja Kluczyk e, Luca Gentilucci b, **, Anna Janecka a, *
a Department of Biomolecular Chemistry, Medical University of Lodz, Mazowiecka 6/8, 92-215, Lodz, Poland
b Department of Chemistry, University of Bologna, Via Selmi 2, 40126, Bologna, Italy
c Scientia Advice, di Roberto Artali, 20832, Desio, Monza and Brianza, Italy
d Institute of Organic Chemistry, Lodz University of Technology, Lodz, Poland
e Faculty of Chemistry, University of Wroclaw, Wroclaw, Poland
ABSTRACT.
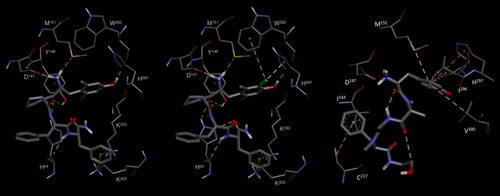
New analogs of the endogenous opioid agonist endomorphin-2 (EM-2, H-Tyr-Pro-Phe-Phe-NH2) have been obtained by introducing modified tyrosines at the position 1 of the sequence. For all analogs, the cis/trans conformation ratio about the tyramine-Pro amide bond, lipophilicity, receptor affinities, and functional activities, have been determined. Among the novel derivatives, [Dmt(30-Cl)]1EM-2 (4) stood out for its subnanomolar m-opioid receptor affinity and potent agonist activity, superior to that of the parent peptide EM-2. Hybrid quantum mechanics/molecular mechanics docking computations supported the cis tyramine-Pro bioactive conformation, and allowed us to analyze the contribution of the substituents of the “message” tyramine to binding, highlighting the role of halogen-bonding in the higher receptor affinity of peptide 4.
J. Org. Chem. 2019, 84, 4992−5004
Rossella De Marco,* Junwei Zhao, Arianna Greco, Simone Ioannone, and Luca Gentilucci*
Department of Chemistry “G. Ciamician”, University of Bologna, via Selmi 2, 40126 Bologna, Italy
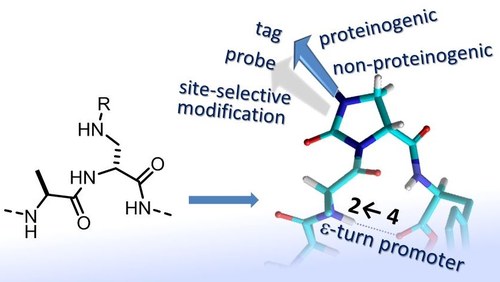
ABSTRACT. Peptidomimetics containing (S)- or (R)-imidazolidin-2-one-4-carboxylate (Imi) have been obtained by the expedient in-peptide cyclization of (S)- or (R)-α,β-diaminopropionic acid (Dap) residues. These Imi scaffolds behave as proline analogues characterized by a flat structure and a trans restricted geometry of the preceding peptide bond and induce well-defined secondary structures in a biomimetic environment. While (S)-Imi peptides adopted a γ′-turn conformation, (R)-Imi induced the contemporary formation of a γ-turn and a rare 11-membered H-bonded structure in the 2→4 opposite direction of the sequence, identified as a ε-turn. In order to exploit these Imi scaffolds as general promoters of unusual secondary structures, proteinaceous side chains have been introduced at the N1 position of the five-membered ring, potentially mimicking any residues. Finally, the Imi rings have been equipped with unnatural side chains or with functionalized substituents, which can be utilized as linkers to chemoselectively bind the Imi-peptides onto nanoparticles, biomaterials, or diagnostic probes.
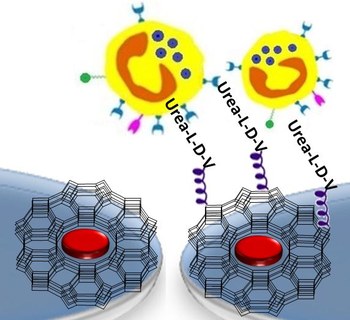
PEPTIDE SCIENCE, 2018, 110 (5), e23081, DOI: 10.1002/bip.23081
Rossella De Marco1 | Arianna Greco1 | Natalia Calonghi2 | Samantha D. Dattoli2 |
Monica Baiula2 | Santi Spampinato2 | Pierre Picchetti3 | Luisa De Cola3,4 |
Michele Anselmi1 | Francesca Cipriani5 | Luca Gentilucci1
1 Department of Chemistry “G. Ciamician”, University of Bologna, via Selmi 2, Bologna 40126, Italy
2 Department of Pharmacy and Biotechnology, University of Bologna, via Irnerio 48, Bologna 40126, Italy
3 Institut de science et d’ingenierie supramoleculaires (ISIS), Universite de Strasbourg and CNR UMR 7006, 8 Allee Gaspard Monge, Strasbourg 67000, France
4 Institut f}ur Nanotechnologie (INT), Karlsruhe Institute of Technology (KIT) - Campus Nord, Hermann-von-Helmholtz-Platz 1, Eggenstein-Leopoldshafen 76344, Germany
5 Department of Medical and Surgical Sciences, University of Bologna, Via Massarenti 11, Bologna 40138, Italy
ABSTRACT. Persistent accumulation of immune cells mediated by alfa4beta1 integrin (VLA-4) is a hallmark of the inflammatory diseases and of chronic inflammation observed in the affected tissues of autoimmune diseases. Aiming at exploring new methods for monitoring the course of the inflammatory processes, we designed the first peptide-functionalized nanostructured devices capable to mimic the high-density multivalency binding between the a4b1 integrin-expressing cells and the ligands overexpressed on the endothelial surfaces, in the proximity of the sites of inflammation. Specifically, we describe the first examples of monolayers constituted by dye-loaded zeolite L crystals, coated with a4b1 integrin peptide ligands, and we analyze the adhesion of model Jurkat cells in comparison to non-alfa4beta1 integrin-expressing cells. In particular, the peptidomimetic diphenylurea-Leu-Asp-Val-diamine allows significant and selective detection of alfa4beta1 integrin-expressing Jurkat cells, after very rapid incubation time, supporting the possible implementation in a diagnostic device capable to detect the desired cells from biological fluids, obtainable from patients in a noninvasive way.