Project “Integrin antagonists as an innovative treatment for age-related macular degeneration”,
2018/0347 financed by Fondazione Cassa di Risparmio in Bologna

Asthma is a chronic inflammatory disease of the airways that causes an increase in bronchial responsiveness which, in turn, causes recurrent episodes of wheezing, chest tightness and coughing. The constant state of inflammation of the airways, which characterizes asthma, leads to an irreversible tissue remodeling, which leads to thickening of the smooth muscle layer (muscle hyperplasia), swollen glands that produce mucus (glandular hyperplasia) and the formation of new blood vessels (angiogenesis). If bronchial asthma left untreated, it can lead to irreversible airway obstruction and even death.
To date, there are not definitive cures for asthma, so everything that it is possible to do is to diagnose and control the disease in medium and long term, reducing the symptoms and preventing acute attacks, in order to preserve respiratory function. Nowadays this is done through the use of different types of drugs, including anti-inflammatory, primarily corticosteroids, and bronchodilators, primarily 2-agonists, that are not free of side effects.
At present there are a limited number of tests available for the diagnosis and monitoring of the disease and for the evaluation the effectiveness of drug treatment. The diagnosis of asthma occurs in fact, generally, once ascertained the symptoms, through the execution of tests to determine the respiratory function of the lungs, such as baseline spirometry and after administration of a bronchodilator, peak expiratory flow monitoring and bronchial challenge test with methacholine. Diagnosis of asthma is made through evaluation of symptoms and pulmonary function testing (PFT) via spirometry. An increase in FEV (forced expiratory volume) of ≥15% in conjunction with reported wheezing, chest tightness, and coughing is diagnostic for asthma. A difficulty arises when patients present with normal spirometry results. Additionally, controlled exacerbation of asthma attacks with methacholine in the clinician's office is a reliable method of eliciting the necessary response to confirm a diagnosis of asthma.
The project AsthmaZoè was aimed at the development of new diagnostic nano-tools for the diagnosis and monitoring of asthma, more efficient, rapid, non-invasive, and reliable. In the last decade, the nanotechnology has seen significant progress and has been extensively studied for potential biomedical applications, including imaging. Nanoparticles are generally not-toxic, and their large surface areas allow for the presentation of large numbers of functional groups, bioactive ligands, imaging devices.
Nanoparticles functionalized with bioactive molecules offer advantages of biocompatibility, biodegradability, and low toxicity compared with synthetic polymers. Rationally designed multifunctionality, such as active targeting or image-contrast enhancement, is one expected characteristic of nanoparticles currently under development. Finally, by engineering their surfaces it is possible to obtain nano-structured devices and microchips composed of monolayers of nanoparticles.
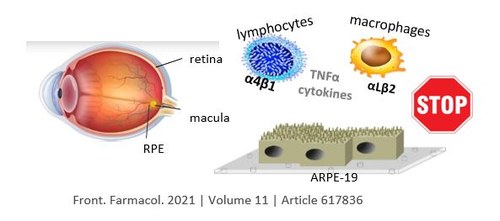
The chronic inflammation that characterizes asthma is mediated by eosinophils, a particular class of leukocytes, normally recruited in the various body districts when it is detected the presence of an exogenous factor, to eliminate it. Very often the progression of asthma is correlated to a increase in circulating and sputum eosinophils; studies (Gibson P G, et al.; Green R H, et al.) indicated that the presence of airway eosinophils is associated with diminished asthma control and increased risk for an exacerbation.
One of the key steps is the interaction that takes place between a specific subclass of transmembrane glycoproteins expressed by eosinophils, known as alfa4beta1 integrins (also known as Very Late Antigen 1, VLA-1), and the Vascular-1 Cell Adhesion Molecules (VCAM-1), small proteins expressed by eosinophils and endothelial cells of affected blood vessels after appropriate stimulation by cytokines. This interaction is essential for the realization of a firm adhesion between the vascular endothelium and leukocyte, followed by the diapedesis of the latter, directed towards the region of inflammation. As a consequence, the introduction of ligands which selectively bind to the integrin receptors onto the nanoparticle surfaces, is expected to consent a specific recognition of the eosinophils.
In conclusion of the project, we successfully designed microchips composed of monolayers of diverse kinds of nanoparticles (nanospheres, or large flat nanochrystals), equipped with ligands of the activated integrins and with diagnostic probes, represent promising diagnostic nanodevices for the non-invasive determination of asthma progression from physiological samples containing activated integrin-expressing eosinophils, such as blood, sputum, or any other obtainable from the patients by conventional methods.
Prof. Luca Gentilucci, Dept. of Chemistry "G. Ciamician", University of Bologna. Role: project manager
Prof. L. Prodi, Dept. of Chemistry "G. Ciamician", University of Bologna. Role: preparation of flurescent PEG-silica nanoparticles.
Prof. L. De Cola, Institut de Science et d'Ingénierie Supramoléculaires (ISIS) Strasbourg (France). Role. Preparation of zeolite nanocrystal nanoparticles.
Prof. S. Schinelli, Dept. of Drug of Science, University of Pavia. Role: in vitro studies.
Prof. Clementina Cocuzza, Department of Surgery and Interdisciplinary medicine, University of Milano-Bicocca. Role: in vivo studies.
Dr.ssa R. De Marco Dept. of Chemistry "G. Ciamician", University of Bologna, Post-Doc fellow. Role: synthesis of peptidomimetic integrin ligands and conjugation to nanoparticles.
Integrin Ligands with alpha/ beta-Hybrid Peptide Structure: Design, Bioactivity, and Conformational Aspects By: De Marco, Rossella; Tolomelli, Alessandra; Juaristi, Eusebio; et al. MEDICINAL RESEARCH REVIEWS, Volume: 36 Issue: 3 Pages: 389-424
Modern tools for the chemical ligation and synthesis of modified peptides and proteins By: Gentilucci, Luca; Tosi, Pierluigi; Bauer, Adriano; et al. FUTURE MEDICINAL CHEMISTRY Volume: 8 Issue: 18 Pages: 2287-2304
Selective detection of α4β1 integrin (VLA-4)-expressing cells using peptide-functionalized nanostructured materials mimicking endothelial surfaces adjacent to inflammatory sites. By De Marco R, Greco A, Calonghi N, Dattoli SD, Baiula M, Spampinato S, Picchetti P, De Cola L, Anselmi M, Cipriani F, Gentilucci L. Biopolymers. 2017 Nov 27. doi: 10.1002/bip.23081. [Epub ahead of print]
DS-70, a novel and potent α4 integrin antagonist, is an effective treatment for experimental allergic conjunctivitis in guinea pigs By Dattoli, Samantha; Baiula, Monica; De Marco, Rossella; Bedini, Andrea; Anselmi, Michele; Gentilucci, Luca; Spampinato, Santi; British Journal of Pharmacology (2018) 175 3891–3910
During the first year we developed a mini-library of minimalist peptides having the acetylated N-terminal. Among the various synthesized compounds, the peptides Ac-D-Trp-PheNH2 (4) and Ac-D-Trp-Phe-GlyNH2 (5) were found to be opioid ligands with nanomolar affinity. Although 4 did not show any agonist activity on MOR, 5 showed a selective, partial MOR agonist. The comparison with structurally related compounds emphasizes the role of D-Trp and reveals that the presence of a basic amino group is not a "conditio sine qua" not for proper interaction and activation of MOR. The most important result achieved during the first year concerns the demonstration that the analgesic activity of short opioid peptides can be separated from the presence of the main pharmacophore group, the protonated amine at physiological pH. This discovery has been demonstrated and developed by us for the first time in the world, and brought to public attention both in the most qualified scientific press (e.g. American Chemical Society) but also in the general press. Peptides 4 and 5 could represent interesting candidates for the development of a new class of lipophilic MOR-active peptidomimetics. The conformational analysis showed that 4 and 5 adopt similar solutions in solution, however 5 tends to bend in a well-defined β-turn, which could contribute to a greater affinity and selectivity. The study of ligand-MOR interaction was carried out in collaboration with Scientia Advice. The molecular docking approach emerges as a powerful tool for discovering new drugs to find and optimize lead compounds. It is a method that estimates the preferred orientations of a drug within its receptor. The results of this computational analysis are much more realistic when the simulations take into account the data provided by experimental methods, in particular SAR, mutagenesis, chimeric receptors, etc. The different classes of ligands, such as morphine and opiates, fentanyl, peptides, peptidomimetics, etc., have different modes of receptor interaction and, for agonists, activation. As a result, a number of specific models have been proposed to explain the link and selectivity of each class. Furthermore, the analysis of ligands without a well-defined message or address, or highly flexible ligands, often leads to alternative models. For example, morphine, the prototypical agonist of MOR, is devoid of a portion of address, and therefore docking analysis has led to different orientations within the receptor cavity. However, some classes of ligands, such as peptidomimetic JOMs, have proven to be very effective in detecting structural requirements for affinity and receptor selectivity, as well as agonism versus antagonism. Furthermore, docking represented a useful technique for the analysis of opioid agonists without the protonable amino group, generally considered as the fundamental pharmacophore interacting with the Asp in TMH3, such as the salvinorins, and the cyclopeptides with β-turn on D -Trp-Phe. In particular, the results could be helpful in revealing the unexpected agonist activity of the natural cyclopeptide CJ-15, 208 and its derivatives.
During the second year of the project we presented the synthesis and pharmacological characterization towards the MOR receptor of a mini-library of tripeptides derived from Ac-D-Trp-PheNH2, free of cationic amino group, containing methyl, nitro, halogen substituents, etc ., in various combinations, on the indole side chain of D-Trp. The replacement strongly impacts receptor affinity, which can vary from none to a nano-molar. Possibly the differences are related to fundamental interactions of pi-stacking hydrogen bond interactions between the ligands and the receptor, while the steric requirements seem less important (see for example the different results for groups of similar size). The quantitative investigation of these interactions and the likely impact in experimentally observed affinity is currently underway in our group. We have also shown that the decoration of peptide structures with different groups influences stability and bioavailability. Peptides with 7-Br-2Me and 5-nitro substituents showed moderately faster analgesia than non-substituent compounds in the tail flick test on mice. These preliminary results support the conjecture that the minimum tripeptides are atypical MOR-selective sequences. Interestingly, the optimality of a three-amino acid motif for biological signaling has been highlighted in a recent article.
Finally, during the second year, we researched the possibility to prepare peptides in environmentally sustainable conditions. The growing need for large quantities of biologically active peptides for the pharmaceutical market has greatly raised the problem of environmental sustainability of their synthesis. In this regard, we have developed a solid phase process in water, using PEG amine resins under controlled conditions, using amino acid carboxyhydride derivatives. This procedure allows to obtain short peptide sequences with reasonable yield and good purity, without the need for coupling reagents or protective groups.
Synthesis of tripeptides containing D-Trp substituted at indole ring, and assessment of MOR affinity and in vivo central antinociception. Rossella De Marco, Andrea Bedini, Santi Spampinato, and Luca Gentilucci. J. Med. Chem. 2014, 57, 6861−6866
Synthesis and Analysis of the Conformational Preferences of 5- Aminomethyloxazolidine-2,4-dione Scaffolds, First Examples of beta2- and beta2,2-homo-Friedinger Lactam Analogs. Arianna Greco, Sara Tani, Rossella De Marco, and Luca Gentilucci. Chem. Eur. J. 2014, 20, 13390 – 13404
Synthesis and assay of retro-alfa4beta1 integrin-targeting motifs. Samantha D. Dattoli, Rossella De Marco, Monica Baiula, Santi Spampinato, Arianna Greco, Alessandra Tolomelli, Luca Gentilucci. European Journal of Medicinal Chemistry 73 (2014) 225-232
In-peptide synthesis of di-oxazolidinone and dehydroamino acid-oxazolidinone motifs as β-turn Inducers. Rossella De Marco, Arianna Greco, Sebastiano Rupiani, Alessandra Tolomelli, Claudia Tomasini, Silvia Pieraccini and Luca Gentilucci. Org. Biomol. Chem., 2013, 11, 4316-4326.
Controlled Solid Phase Peptide Bond Formation Using N-Carboxyanhydrides and PEG Resins in Water. Rossella De Marco, Alessandra Tolomelli, Arianna Greco, Luca Gentilucci. ACS Sus. Chem. Eng. 2013, 1, 566-569
Addressing the Interactions between Opioid Ligands and Their Receptors Classic and Nonclassic Cases. Luca Gentilucci, capitol for the book Neuropeptides in Neuroprotection and Neuroregeneration, published in 2012 by Taylor & Francis Group, LLC.
Opioid Activity Profiles of Oversimplified Peptides Lacking in the Protonable N-Terminus. Rossella De Marco, Alessandra Tolomelli, Santi Spampinato, Andrea Bedini, and Luca Gentilucci. J. Med. Chem. 2012, 55, 10292-10296
Molecular Docking of Opiates and Opioid Peptides, a Tool for the Design of Selective Agonists and Antagonists, and for the Investigation of Atypical Ligand-Receptor Interactions. L. Gentilucci, A. Tolomelli, R. De Marco, and R. Artali. Current Medicinal Chemistry, 2012, 19, 1587-1601