Publications
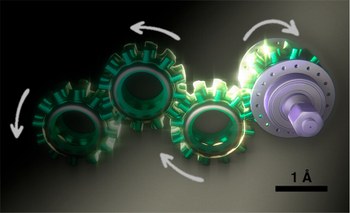
Photoactivated artificial molecular motors
JACS Au
Stefano Corra, Massimiliano Curcio, Alberto Credi
Published online: 8 May 2023
2023, Vol. 3, in press
DOI: 10.1021/jacsau.3c00089
Accurate control of long-range motion at the molecular scale holds great potential for the development of ground-breaking applications in energy storage and bionanotechnology. The past decade has seen tremendous development in this area, with a focus on the directional operation away from thermal equilibrium, giving rise to tailored man-made molecular motors. As light is a highly tunable, controllable, clean, and renewable source of energy, photochemical processes are appealing to activate molecular motors. Nonetheless, the successful operation of molecular motors fueled by light is a highly challenging task, which requires a judicious coupling of thermal and photoinduced reactions. In this paper, we focus on the key aspects of light-driven artificial molecular motors with the aid of recent examples. A critical assessment of the criteria for the design, operation, and technological potential of such systems is provided, along with a perspective view on future advances in this exciting research area.
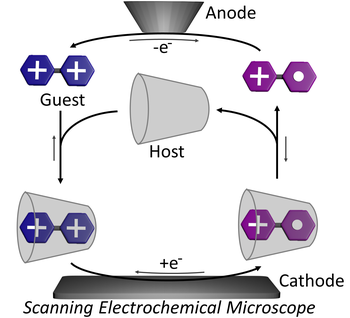
Autonomous non-equilibrium self-assembly and molecular movements powered by electrical energy
Angewandte Chemie International Edition
Giulio Ragazzon, Marco Malferrari, Arturo Arduini, Andrea Secchi, Stefania Rapino, Serena Silvi, Alberto Credi
Published online: 24 November 2022
2023, Vol. 62, e202214265
DOI: 10.1002/anie.202214265
The ability to exploit energy autonomously is one of the hallmarks of Life. Mastering such processes in artificial nanosystems can open technological opportunities. In the last decades, light- and chemically-driven autonomous systems have been developed in relation to conformational motion and self-assembly, mostly in relation to molecular motors. On the contrary, despite electrical energy is an attractive energy source to power nanosystems, its autonomous harnessing received little attention. Herein we consider an operation mode allowing the autonomous exploitation of electrical energy by a self-assembling system. Threading and dethreading motions of a pseudorotaxane take place autonomously in solution, powered by the current flowing between the electrodes of a scanning electrochemical microscope. The underlying autonomous energy ratchet mechanism drives the self-assembly steps away from equilibrium with a higher energy efficiency compared to other autonomous systems. The strategy is general and might be extended to other redox-driven systems.
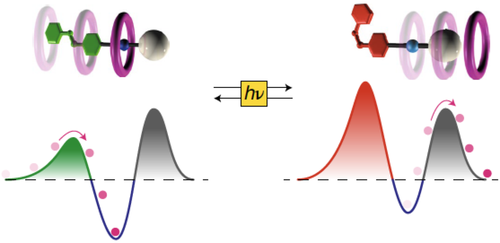
Kinetic and energetic insights into the dissipative non-equilibrium operation of an autonomous light-powered supramolecular pump
Nature Nanotechnology
Stefano Corra, Marina Tranfić Bakić, Jessica Groppi, Massimo Baroncini, Serena Silvi, Emanuele Penocchio, Massimiliano Esposito, Alberto Credi
Published online: 27 June 2022
2022, Vol. 17, pp. 746-751
DOI: 10.1038/s41565-022-01151-y
Natural and artificial autonomous molecular machines operate by constantly dissipating energy coming from an external source to maintain a non-equilibrium state. Quantitative thermodynamic characterization of these dissipative states is highly challenging as they exist only as long as energy is provided. Here we report on the detailed physicochemical characterization of the dissipative operation of a supramolecular pump. The pump transduces light energy into chemical energy by bringing self-assembly reactions to non-equilibrium steady states. The composition of the system under light irradiation was followed in real time by 1H NMR for four different irradiation intensities. The experimental composition and photon flow were then fed into a theoretical model describing the non-equilibrium dissipation and the energy storage at the steady state. We quantitatively probed the relationship between the light energy input and the deviation of the dissipative state from thermodynamic equilibrium in this artificial system. Our results provide a testing ground for newly developed theoretical models for photoactivated artificial molecular machines operating away from thermodynamic equilibrium.
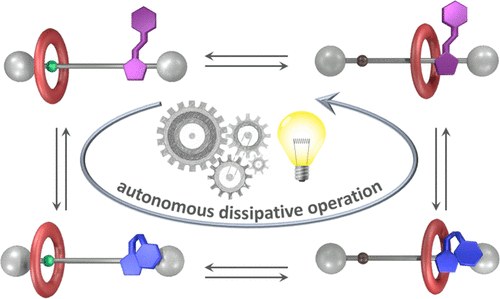
Photoinduced autonomous nonequilibrium operation of a molecular shuttle by combined isomerization and proton transfer through a catalytic pathway
Journal of the American Chemical Society
Federico Nicoli, Massimiliano Curcio, Marina Tranfić Bakić, Erica Paltrinieri, Serena Silvi, Massimo Baroncini, Alberto Credi
Published online: 16 May 2022
2022, Vol. 144, pp. 10180-10185
DOI: 10.1021/jacs.1c13537
We describe a [2]rotaxane whose recognition sites for the ring are a dibenzylammonium moiety, endowed with acidic and H-bonding donor properties, and an imidazolium center bearing a photoactive phenylazo substituent. Light irradiation of this compound triggers a network of E/Z isomerization and proton transfer reactions that enable autonomous and reversible ring shuttling away from equilibrium.
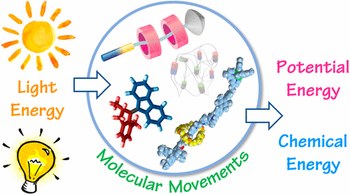
Photochemical energy conversion with artificial molecular machines
ACS Energy & Fuels
Leonardo Andreoni, Massimo Baroncini, Serena Silvi, Chiara Taticchi, Alberto Credi
Published online: 1 October 2021
2021, Vol. 35, pp. 18900-18914
DOI: 10.1021/acs.energyfuels.1c02921
The exploitation of sunlight as a clean, renewable, and distributed energy source is key to facing the energetic demand of modern society in a sustainable and affordable fashion. In the past few decades, chemists have learned to make molecular machines, that is, synthetic chemical systems in which energy inputs cause controlled movements of molecular components that could be used to perform a task. A variety of artificial molecular machines operated by light have been constructed by implementing photochemical processes within appropriately designed (supra)molecular assemblies. These studies could open up new routes for the realization of nanostructured devices and materials capable to harness, convert, and store light energy.
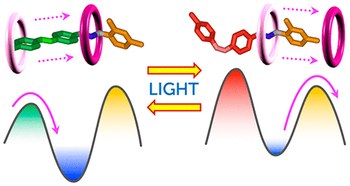
Second-generation light-fueled supramolecular pump
Journal of the American Chemical Society
Martina Canton, Jessica Groppi, Lorenzo Casimiro, Stefano Corra, Massimo Baroncini, Serena Silvi, Alberto Credi
Published online: 20 July 2021
2021, Vol. 143, pp. 10890-10894
DOI: 10.1021/jacs.1c06027
We describe the modular design of a pseudorotaxane-based supramolecular pump and its photochemically driven autonomous nonequilibrium operation in a dissipative regime. These properties derive from careful engineering of the energy maxima and minima along the threading coordinate and their light-triggered modulation. Unlike its precursor, this second-generation system is amenable to functionalization for integration into more complex devices.
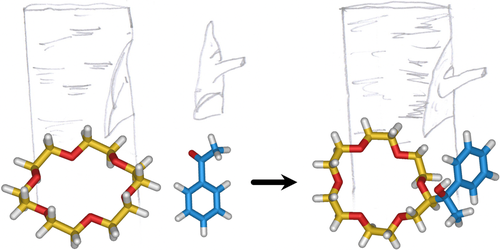
Direct synthetic routes to functionalised crown ethers
Organic Chemistry Frontiers
Federico Nicoli, Massimo Baroncini, Serena Silvi, Jessica Groppi, Alberto Credi
Published online: 1 July 2021
2021, Vol. 8, pp. 5531-5549
DOI: 10.1039/D1QO00699A
Crown ethers are macrocyclic hosts that can complex a wide range of inorganic and organic cations as well as neutral guest species. Their widespread utilization in several areas of fundamental and applied chemistry strongly relies on strategies for their functionalisation, in order to obtain compounds that could carry out multiple functions and could be incorporated in sophisticated systems. Although functionalised crown ethers are normally synthesised by templated macrocyclisation using appropriately substituted starting materials, the direct addition of functional groups onto a pre-formed macrocyclic framework is a valuable yet underexplored alternative. Here we review the methodologies for the direct functionalisation of aliphatic and aromatic crown ethers sporadically reported in the literature over a period of four decades. The general approach for the introduction of moieties on aliphatic crown ethers involves a radical mediated cross dehydrogenative coupling initiated either by photochemical or thermal/chemical activation, while aromatic crown ethers are commonly derivatised via electrophilic aromatic substitution. Direct functionalization routes can reduce synthetic effort, allow the later modification of crown ether-based architectures, and disclose new ways to exploit these versatile macrocycles in contemporary supramolecular science and technology.
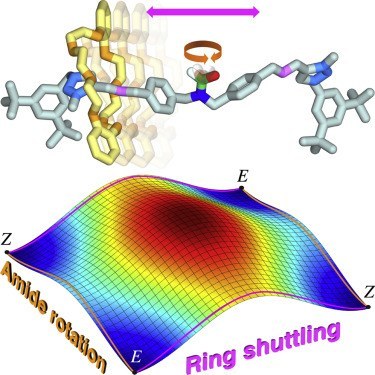
Stereodynamics of E/Z isomerization in rotaxanes through mechanical shuttling and covalent bond rotation
Chem
Stefano Corra, Christiaan de Vet, Massimo Baroncini, Alberto Credi, Serena Silvi
Published online: 17 May 2021
2021, Vol. 7, pp. 2137-2150
DOI: 10.1016/j.chempr.2021.04.010
The mechanical bond has opened a new world for structural and dynamic stereochemistry, which is still largely underexplored and whose significance for various applications is becoming increasingly evident. We demonstrate that designed rearrangements involving both covalent and mechanical bonds can be integrated in [2]rotaxanes, leading to interesting consequences in terms of E/Z isomerization mechanisms. Two entirely distinct and concomitant stereomutations, pertaining to the same stereogenic element but involving different kinds of linkages within the molecule, are observed and are thoroughly characterized. The rate of the two processes is affected in opposite ways upon changing solvent polarity; such a phenomenon can be used to selectively modify the rate of each motion and adjust the relative contribution of the two mechanisms to the isomerization. Although the movements are not synchronized, an analysis of the intriguing fundamental implications for transition state theory, reaction pathway bifurcation, and microscopic reversibility was triggered by our experimental observations.

Artificial supramolecular pumps powered by light
Chemistry - A European Journal
Stefano Corra, Lorenzo Casimiro, Massimo Baroncini, Jessica Groppi, Marcello La Rosa, Marina Tranfic Bakic, Serena Silvi, Alberto Credi
Published online: 5 May 2021
2021, Vol. 27, pp. 11076-11083
DOI: 10.1002/chem.202101163
The development of artificial nanoscale motors that can use energy from a source to perform tasks requires systems capable of performing directionally controlled molecular movements and operating away from chemical equilibrium. Here we describe the design, synthesis and properties of pseudorotaxanes in which a photon input triggers the unidirectional motion of a macrocyclic ring with respect to a non‐symmetric molecular axle. The photoinduced energy ratcheting at the basis of the pumping mechanism is validated by measuring the relevant thermodynamic and kinetic parameters. Owing to the photochemical behaviour of the azobenzene moiety embedded in the axle, the pump can repeat its operation cycle autonomously under continuous illumination. We use NMR spectroscopy to observe the dissipative non‐equilibrium state generated in situ by light irradiation. We also show that fine changes in the axle structure lead to an improvement in the performance of the motor. Such results highlight the modularity and versatility of this minimalist pump design, which provides facile access to dynamic systems that operate under photoinduced non‐equilibrium regimes.
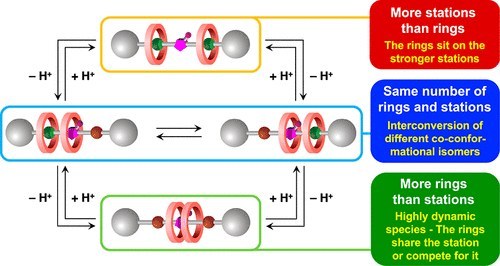
Chemically induced mismatch of rings and stations in [3]rotaxanes
Journal of the American Chemical Society
Massimiliano Curcio, Federico Nicoli, Erica Paltrinieri, Ettore Fois, Gloria Tabacchi, Luigi Cavallo, Serena Silvi, Massimo Baroncini, Alberto Credi
Published online: 29 April 2021
2021, Vol. 143, pp. 8046-8055
DOI: 10.1021/jacs.1c02230
The mechanical interlocking of molecular components can lead to the appearance of novel and unconventional properties and processes, with potential relevance for applications in nanoscience, sensing, catalysis, and materials science. We describe a [3]rotaxane in which the number of recognition sites available on the axle component can be changed by acid–base inputs, encompassing cases in which this number is larger, equal to, or smaller than the number of interlocked macrocycles. These species exhibit very different properties and give rise to a unique network of acid–base reactions that leads to a fine pKa tuning of chemically equivalent acidic sites. The rotaxane where only one station is available for two rings exhibits a rich coconformational dynamics, unveiled by an integrated experimental and computational approach. In this compound, the two crown ethers compete for the sole recognition site, but can also come together to share it, driven by the need to minimize free energy without evident inter-ring interactions.
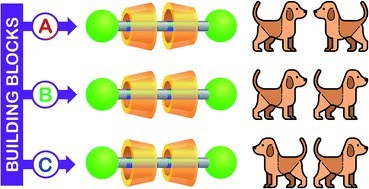
Selective access to constitutionally identical, orientationally isomeric calix[6]arene-based [3]rotaxanes by an active template approach
Chemical Science
Margherita Bazzoni, Leonardo Andreoni, Serena Silvi, Alberto Credi, Gianpiero Cera, Andrea Secchi, Arturo Arduini
Published online: 1 April 2021
2021, Vol. 12, pp. 6419-6428
DOI: 10.1039/d1sc00279a
Tris(phenylureido)calix[6]arene is endowed with unique properties that make it a valuable macrocyclic component for the synthesis of mechanically interlocked molecules. Its three-dimensional and intrinsically nonsymmetric structure is kinetically selective toward two processes: (i) in apolar media, the threading of bipyridinium based axle-like components takes place exclusively from the upper rim; (ii) SN2 alkylation reactions of a pyridylpyridinium precursor engulfed in the cavity occur selectively at pyridylpyridinium nitrogen atom located at the macrocycle upper rim (active template synthesis). Here we exploit such properties to prepare two series of [3]rotaxanes, each consisting of three sequence isomers that arise from the threading of two identical but nonsymmetric wheels on a symmetric thread differing only for the reciprocal orientation of the macrocycles. The features of the calix[6]arene and the active template synthetic approach, together with a careful selection of the precursors, enabled us to selectively synthesise the [3]rotaxane sequence isomers of each series with fast kinetics and high yields.
Heteroditopic calix[6]arene based intervowen and interlocked molecular devices
The Chemical Record
Gianpiero Cera, Arturo Arduini, Andrea Secchi, Alberto Credi, Serena Silvi
Published online: 3 March 2021
2021, Vol. 21, pp. 1161-1181
DOI: 10.1002/tcr.202100012
This personal account summarizes the achievement gained during the last two decades on the use of the calix[6]arene as a platform to build‐up interwoven and interlocked structures for the synthesis of oriented (pseudo)rotaxanes. We also account on how these calix[6]arene hosts affect the reactivity or spectroscopic properties of their bound guests.
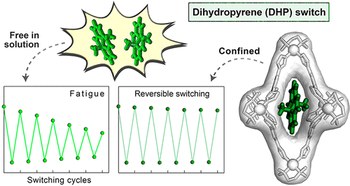
Improving fatigue resistance of dihydropyrene by encapsulation within a coordination cage
Journal of the American Chemical Society
Martina Canton, Angela B. Grommet, Luca Pesce, Julius Gemen, Shiming Li, Yael Diskin-Posner, Alberto Credi, Giovanni M. Pavan, Joakim Andréasson, Rafal Klajn
Published online: 14 August 2020
2020, Vol. 142, pp. 14557-14565
DOI: 10.1021/jacs.0c06146
Photochromic molecules undergo reversible isomerization upon irradiation with light at different wavelengths, a process that can alter their physical and chemical properties. For instance, dihydropyrene (DHP) is a deep-colored compound that isomerizes to light-brown cyclophanediene (CPD) upon irradiation with visible light. CPD can then isomerize back to DHP upon irradiation with UV light or thermally in the dark. Conversion between DHP and CPD is thought to proceed via a biradical intermediate; bimolecular events involving this unstable intermediate thus result in rapid decomposition and poor cycling performance. Here, we show that the reversible isomerization of DHP can be stabilized upon confinement within a PdII6L4 coordination cage. By protecting this reactive intermediate using the cage, each isomerization reaction proceeds to higher yield, which significantly decreases the fatigue experienced by the system upon repeated photocycling. Although molecular confinement is known to help stabilize reactive species, this effect is not typically employed to protect reactive intermediates and thus improve reaction yields. We envisage that performing reactions under confinement will not only improve the cyclic performance of photochromic molecules, but may also increase the amount of product obtainable from traditionally low-yielding organic reactions.
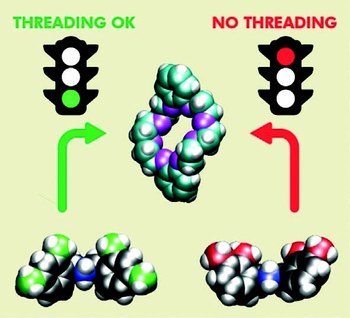
Precision molecular threading/dethreading
Angewandte Chemie International Edition
Jessica Groppi, Lorenzo Casimiro, Martina Canton, Stefano Corra, Mina Jafari-Nasab, Gloria Tabacchi, Luigi Cavallo, Massimo Baroncini, Serena Silvi, Ettore Fois, Alberto Credi
Published online: 12 May 2020
2020, Vol. 59, pp. 14825-14834
DOI: 10.1002/anie.202003064
The general principles guiding the design of molecular machines based on interlocked structures are well known. Nonetheless, the identification of suitable molecular components for a precise tuning of the energetic parameters that determine the mechanical link is still challenging. Indeed, what are the reasons of the “all‐or‐nothing” effect, which turns a molecular “speed‐bump” into a stopper in pseudorotaxane‐based architectures? Here we investigate the threading and dethreading processes for a representative class of molecular components, based on symmetric dibenzylammonium axles and dibenzo[24]crown‐8 ether, with a joint experimental‐computational strategy. From the analysis of quantitative data and an atomistic insight, we derive simple rules correlating the kinetic behaviour with the substitution pattern, and provide rational guidelines for the design of modules to be integrated in molecular switches and motors with sophisticated dynamic features.

Molecular machines
Alberto Credi, Vincenzo Balzani
1088press
88 pages
Alma Mater Studiorum - Università di Bologna
Published online: April 2020
ISBN: 978-88-31926-18-8
ISBN-A: 10.978.8831926/188
DOI: 10.12878/1088pressbit2020_1
The design and construction of machines and motors of molecular size is a stimulating scientific challenge and a primary objective of nanotechnology. During the past thirty years, chemists have taken up this challenge and learned how to make and operate simple nanoscale machines. Although these tiny devices are not yet part of our everyday life, we are approaching a new industrial revolution that opens up radically new perspectives for applications in catalysis, smart materials, robotics, information technology, and medical diagnostics and therapy.
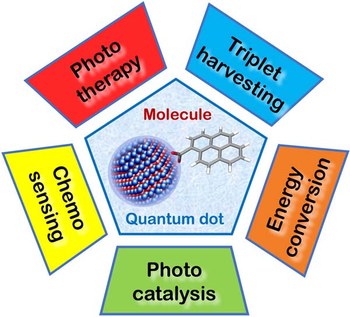
Semiconductor quantum dots as components of photoactive supramolecular architectures
ChemistryOpen
Marcello La Rosa, Emily H. Payne, Alberto Credi
Published online: 10 February 2020
2020, Vol. 9, Issue 2, pp. 200-213
DOI: 10.1002/open.201900336
Luminescent quantum dots (QDs) are colloidal semiconductor nanocrystals consisting of an inorganic core covered by a molecular layer of organic surfactants. Although QDs have been known for more than thirty years, they are still attracting the interest of researchers because of their unique size‐tunable optical and electrical properties arising from quantum confinement. Moreover, the controlled decoration of the QD surface with suitable molecular species enables the rational design of inorganic‐organic multicomponent architectures that can show a vast array of functionalities. This minireview highlights the recent progress in the use of surface‐modified QDs – in particular, those based on cadmium chalcogenides – as supramolecular platforms for light‐related applications such as optical sensing, triplet photosensitization, photocatalysis and phototherapy.
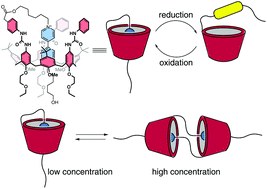
Synthesis and properties of a redox-switchable calix[6]arene-based molecular lasso
Organic Chemistry Frontiers
Guido Orlandini, Lorenzo Casimiro, Margherita Bazzoni, Beatrice Cogliati, Alberto Credi, Marco Lucarini, Serena Silvi, Arturo Arduini, Andrea Secchi
Published online: 30 December 2019
2020, Vol. 7, pp. 648-659
DOI: 10.1039/c9qo01379b
The synthesis and characterisation of calix[6]arene-based lasso-like molecular structures is described. These interwoven structures consist of an electrochemical responsive N,N′-dialkylviologen arm covalently anchored at the upper rim of a triphenylureido calix[6]arene-based wheel. Upon reduction of the viologen core, a hollow tridimensional macrocyclic structure can be generated. This process is reversible, and the original lasso-like structure can be regenerated by oxidizing the viologen arm to its original dicationic form. Electrochemical and EPR techniques investigated the ability of the system to perform threading/dethreading movements upon redox switching. The functionalisation of the arm ω-hydroxy ending with a bulky diphenylacetyl group converts the self-threaded structure in a blocked interwoven molecular compound belonging to the class of [1]rotaxanes. The ability to form dimeric structures in the shape of a [c2]daisy chain was also demonstrated, an unprecedented result for calixarene macrocycles.

Macchine molecolari
Ithaca-Viaggio nella Scienza
Alberto Credi, Vincenzo Balzani
Published online: 1 January 2020
2020, Vol. XIV, pp. 5-17
La costruzione di macchine di dimensioni molecolari è uno straordinario risultato scientifico e un obiettivo primario della nanotecnologia. Nel corso degli ultimi trent’anni, i chimici in varie parti del mondo hanno imparato a realizzare delle semplici macchine nanometriche. Anche se questi minuscoli dispositivi non sono ancora divenuti parte integrante della nostra vita quotidiana, siamo ormai alle soglie di una nuova rivoluzione industriale, capace di cambiare il nostro futuro con applicazioni innovative nella tecnologia dei materiali, nell’informatica, nella robotica e nella medicina.

Light-responsive (supra)molecular architectures: recent advances
Advanced Optical Materials
Massimo Baroncini, Jessica Groppi, Stefano Corra, Serena Silvi, Alberto Credi
First published: 17 May 2019
2019, Vol. 7, Issue 16, art. n.1900392, pp. 1-16
DOI: 10.1002/adom.201900392
The development and investigation of (supra)molecular‐based architectures characterized by light‐activated functionalities is a highly relevant topic of chemical research. The interest on photo‐controlled systems arises not only from their potential applications in different fields of technology but also from their scientific significance related to the understanding of light–matter interactions at the nanoscale. Indeed, light is a peculiar and unique tool as it can be conveniently applied to supply the energy required to affect and operate a system and, at the same time, to probe its state and investigate its transformations. Some basic aspects of light‐induced processes in (supra)molecular architectures are discussed here in the frame of their use to implement novel functionalities in nanostructured systems and materials. In this perspective, a few recent examples from our own work will be illustrated which are meant to provide an overview of the current directions in this highly cross‐disciplinary field of research.
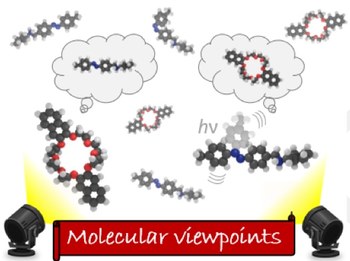
Individual‐molecule perspective analysis of chemical reaction networks: the case of a light‐driven supramolecular pump
Angewandte Chemie International Edition
Andrea Sabatino, Emanuele Penocchio, Giulio Ragazzon, Alberto Credi, Diego Frezzato
Published online: 4 August 2019
2019, Vol. 58, pp. 14341-14348
DOI: 10.1002/anie.201908026
The first study in which stochastic simulations of a two‐component molecular machine are performed in the mass‐action regime is presented. This system is an autonomous molecular pump consisting of a photoactive axle that creates a directed flow of rings through it by exploiting light energy away from equilibrium. The investigation demonstrates that the pump can operate in two regimes, both experimentally accessible, in which light‐driven steps can be rate‐determining or not. The number of photons exploited by an individual molecular pump, as well as the precision of cycling and the overall efficiency, critically rely on the operating regime of the machine. This approach provides useful information not only to guide the chemical design of a self‐assembling molecular device with desired features, but also to elucidate the effect of the environment on its performance, thus facilitating its experimental investigation.
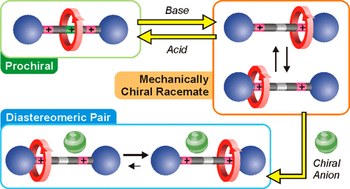
Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]rotaxane molecular shuttle
Journal of the American Chemical Society
Stefano Corra, Christiaan de Vet, Jessica Groppi, Marcello La Rosa, Serena Silvi, Massimo Baroncini, Alberto Credi
Published online: 25 May 2019
2019, Vol. 141, pp. 9129-9133
DOI: 10.1021/jacs.9b00941
We exploit a reversible acid-base triggered molecular shuttling process to switch an appropriately designed rotaxane between prochiral and mechanically planar chiral forms. The mechanically planar enantiomers and their interconversion, arising from ring shuttling, have been characterized by NMR spectroscopy. We also show that the supramolecular interaction of the positively charged rotaxane with optically active anions causes an imbalance in the population of the two enantiomeric coconformations. This result represents an unprecedented example of chiral molecular recognition and can disclose innovative approaches to enantioselective sensing and catalysis.
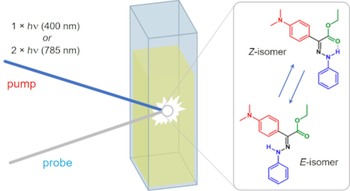
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein Journal of Organic Chemistry
Alessandro Iagatti, Baihao Shao, Alberto Credi, Barbara Ventura, Ivan Aprahamian, Mariangela Di Donato
Published online: 15 October 2019
2019, Vol. 15, pp. 2438-2446
DOI: 10.3762/bjoc.15.236
In this work we apply a combination of steady state and time resolved luminescence and absorption spectroscopies to investigate the excited-state dynamics of a recently developed molecular photoswitch, belonging to the hydrazone family. The outstanding properties of this molecule, involving fluorescence toggling, bistability, high isomerization quantum yield and non-negligible two-photon absorption cross section, make it very promising for numerous applications. Here we show that the light induced Z/E isomerization occurs on a fast <1 ps timescale in both toluene and acetonitrile, while the excited state lifetime of the Z-form depends on solvent polarity, suggesting a partial charge transfer nature of its low lying excited state. Time-resolved luminescence measurements evidence the presence of a main emission component in the 500–520 nm spectral range, attributed to the Z-isomer, and a very short living blue-shifted emission, attributed to the E-isomer. Finally, transient absorption measurements performed upon far-red excitation are employed as an alternative method to determine the two-photon absorption cross-section of the molecule.
Photo- and redox-driven artificial molecular motors
Chemical Reviews
Massimo Baroncini, Serena Silvi, Alberto Credi
Published online: 15 August 2019
2020, Vol.120, pp. 200-268
DOI: 10.1021/acs.chemrev.9b00291
Directed motion at the nanoscale is a central attribute of life, and chemically driven motor proteins are nature’s choice to accomplish it. Motivated and inspired by such bionanodevices, in the past few decades chemists have developed artificial prototypes of molecular motors, namely, multicomponent synthetic species that exhibit directionally controlled, stimuli-induced movements of their parts. In this context, photonic and redox stimuli represent highly appealing modes of activation, particularly from a technological viewpoint. Here we describe the evolution of the field of photo- and redox-driven artificial molecular motors, and we provide a comprehensive review of the work published in the past 5 years. After an analysis of the general principles that govern controlled and directed movement at the molecular scale, we describe the fundamental photochemical and redox processes that can enable its realization. The main classes of light- and redox-driven molecular motors are illustrated, with a particular focus on recent designs, and a thorough description of the functions performed by these kinds of devices according to literature reports is presented. Limitations, challenges, and future perspectives of the field are critically discussed.
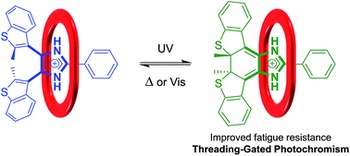
Threading-gated photochromism in [2]pseudorotaxanes
Chemical Science
Giorgio Baggi, Lorenzo Casimiro, Massimo Baroncini, Serena Silvi, Alberto Credi, Stephen J. Loeb
Published online: 23 April 2019
2019, Vol. 10, pp. 5104-5113
DOI: 10.1039/c9sc00913b
Rigid, Y-shaped imidazole compounds containing the bis(thienyl)ethene moiety were designed and synthesized. The 4,5-bis(benzothienyl)-2-phenylimidazolium cations were then used as axles for [2]pseudorotaxane formation with 24-membered crown ether wheels. It was demonstrated using 1H NMR spectroscopy, UV-Vis absorption and emission spectroscopies that this host-guest interaction results in significant changes in the photochromic properties of the imidazolium axles. This is a rare example of gated photochromism, which exploits the recognition event of an interpenetrated molecular system to tune the photochromic properties in one of the components.
New geometries for calix[6]arene-based rotaxanes
European Journal of Organic Chemistry
Margherita Bazzoni, Valeria Zanichelli, Lorenzo Casimiro, Chiara Massera, Alberto Credi, Andrea Secchi, Serena Silvi, Arturo Arduini
Published online: 22 March 2019
2019, pp. 3513-3124
DOI: 10.1002/ejoc.201900211
Understanding the role played by the nature, number and arrangement of binding sites anchored to a macrocycle remains a topic of current interest for the synthesis of new interwoven species. We report the synthesis and detailed structural characterisation of a new calix[6]arene derivative decorated with two phenylureido groups anchored at the diametrical position of the calix upper rim that adopts a 1,2,3-alternate conformation in solution and in the solid state. Preliminary data on the ability of this host to form redox-active pseudorotaxanes and rotaxanes are reported.
Photoactive molecular-based devices, machines and materials: recent advances
European Journal of Inorganic Chemistry
Massimo Baroncini, Martina Canton, Lorenzo Casimiro, Stefano Corra, Jessica Groppi, Marcello La Rosa, Serena Silvi, Alberto Credi
Published online: 14 September 2018
2018, pp. 4589-4603
DOI: 10.1002/ejic.201800923
Molecular and supramolecular-based systems and materials that can perform predetermined functions in response to light stimulation have been extensively studied in the past three decades. Their investigation continues to be a highly stimulating topic of chemical research, not only because of the inherent scientific value related to a bottom-up approach to functional nanostructures, but also for the prospective applications in diverse fields of technology and medicine. Light is an important tool in this context, as it can be conveniently used both for supplying energy to the system and for probing its states and transformations. In this microreview we recall some basic aspects of light-induced processes in (supra)molecular assemblies, and discuss their exploitation to implement novel functionalities with nanostructured devices, machines and materials. To this aim we illustrate a few examples from our own recent work, which are meant to illustrate the trends of current research in the field.

Reversible photoswitching and isomer-dependent diffusion of single azobenzene tetramers on a metal surface
Angewandte Chemie International Edition
Christophe Nacci, Massimo Baroncini, Alberto Credi, Leonhard Grill
Published online: 6 September 2018
2018, Vol. 57, pp. 15034-15039
DOI: 10.1002/anie.201806536
Azobenzene is a prototypical molecular switch that can be reversibly photoisomerized between the nearly planar and apolar trans form, and the distorted, polar cis form. Thus far, most studies related to azobenzene derivatives have focused on planar adsorbed molecules. We present here the study of a three‐dimensional shape‐persistent molecular architecture consisting of four tetrahedrally arranged azobenzene units and adsorbed on a Ag(111) surface. While the azobenzenes of the tripod in contact with the surface lose their switching ability, different isomers of the upright standing arm of the tetramer are obtained reversibly and efficiently by illumination at different wavelengths, revealing time constants of only a few minutes. Diffusion on the surface turns out to be dependent on the isomeric state ‐ trans or cis ‐ of the upright oriented azobenzene group. Hence, molecular mobility can be modulated via their isomeric state, which suggests that for instance molecular growth processes could be controlled by external stimuli.

A molecular cable car for transmembrane ion transport
Angewandte Chemie International Edition
Alberto Credi
Published online: 20 February 2019
2019, Vol. 58, pp. 4108-4110
DOI: 10.1002/anie.201814333
The controlled transport of molecular and ionic substrates across bilayer membranes is a fundamental task for the operation of living organisms. It is also a highly fascinating and demanding challenge for artificial molecular machines. The recent report of a synthetic transmembrane molecular shuttle that can transport potassium ions selectively down a gradient in a liposomal system makes a small but significant step forward towards this goal
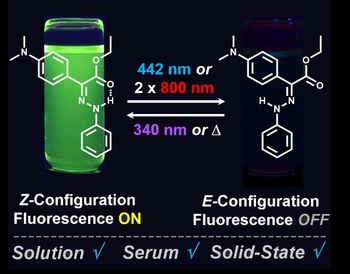
Solution and solid-state emission toggling of a photochromic hydrazone
Journal of the American Chemical Society
Baihao Shao, Massimo Baroncini, Hai Qian, Laura Bussotti, Mariangela Di Donato, Alberto Credi, Ivan Aprahamian
Published online: 25 September 2018
2018, Vol. 140, pp. 12323-12327
DOI: 10.1021/jacs.8b07108
The proliferation of light-activated switches in recent years has enabled their use in a broad range of applications encompassing an array of research fields and disciplines. All current systems, however, have limitations (e.g., from complicated synthesis to incompatibility in biologically relevant media and lack of switching in the solid-state) that can stifle their real-life application. Here we report on a system that packs most, if not all, the desired, targeted and sought-after traits from photochromic compounds (bistability, switching in various media ranging from serum to solid-state, while exhibiting ON/OFF fluorescence emission switching, and two-photon assisted near infrared light toggling) in an easily accessible structure.
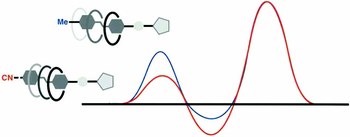
Photochemical investigation of cyanoazobenzene derivatives as components of artificial supramolecular pumps
Photochemical & Photobiological Sciences
Lorenzo Casimiro, Jessica Groppi, Massimo Baroncini, Marcello La Rosa, Alberto Credi, Serena Silvi
Published online: 3 May 2018
2018, Vol. 17, Issue 6, pp. 734-740
DOI: 10.1039/c8pp00062j
Among the plethora of photochromes reported so far, azobenzene has been proven to be the most suitable photoswitch for molecular systems and materials, due to its highly efficient and clean E–Z photoisomerization. Here we report two ammonium-based molecular axles bearing one or two p-cyanoazobenzene units at the extremities, able to form pseudorotaxanes with a crown ether macrocycle. The photochemistry of these compounds was studied in the isolated forms and in the pseudorotaxanes, showing that the functionalization speeds up the threading process without affecting the photochemical properties of the system. These results suggest that the investigated pseudorotaxanes can form the basis of new prototypes of artificial molecular-level pumps.
Electrochemically triggered co-conformational switching in a [2]catenane comprising a non-symmetric calix[6]arene wheel and a two-station oriented macrocycle
Molecules
Valeria Zanichelli, Luca Dallacasagrande, Arturo Arduini, Andrea Secchi, Giulio Ragazzon, Serena Silvi, Alberto Credi
Published online: 11 May 2018
Vol. 23, Issue 5, art. n. 1156, pp. 1-12
DOI: 10.3390/molecules23051156
Catenanes with desymmetrized ring components can undergo co-conformational rearrangements upon external stimulation and can form the basis for the development of molecular rotary motors. We describe the design, synthesis and properties of a [2]catenane consisting of a macrocycle—the ‘track’ ring—endowed with two distinct recognition sites (a bipyridinium and an ammonium) for a calix[6]arene—the ‘shuttle’ ring. By exploiting the ability of the calixarene to thread appropriate non-symmetric axles with directional selectivity, we assembled an oriented pseudorotaxane and converted it into the corresponding oriented catenane by intramolecular ring closing metathesis. Cyclic voltammetric experiments indicate that the calixarene wheel initially surrounds the bipyridinium site, moves away from it when it is reduced, and returns in the original position upon reoxidation. A comparison with appropriate model compounds shows that the presence of the ammonium station is necessary for the calixarene to leave the reduced bipyridinium site.
Making and operating molecular machines: a multidisciplinary challenge
ChemistryOpen
Massimo Baroncini, Lorenzo Casimiro, Christiaan de Vet, Jessica Groppi, Serena Silvi, Alberto Credi
Published online: 2 February 2018
2018, Vol. 7, pp. 169-179
DOI: 10.1002/open.201700181
Movement is one of the central attributes of life, and a key feature in many technological processes. While artificial motion is typically provided by macroscopic engines powered by internal combustion or electrical energy, movement in living organisms is produced by machines and motors of molecular size that typically exploit the energy of chemical fuels at ambient temperature to generate forces and ultimately execute functions. The progress in several areas of chemistry, together with an improved understanding of biomolecular machines, has led to the development of a large variety of wholly synthetic molecular machines. These systems have the potential to bring about radical innovations in several areas of technology and medicine. In this article we discuss, with the help of a few examples, the multidisciplinary aspects of research on artificial molecular machines, and highlight its translational character.
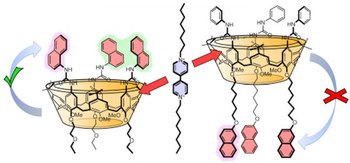
Plugging a bipyridinium axle into multichromophoric calix[6]arene wheels bearing naphthyl units at different rims
ChemistryOpen
Guido Orlandini, Giulio Ragazzon, Valeria Zanichelli, Lorenzo Degli Esposti, Massimo Baroncini, Serena Silvi, Margherita Venturi, Alberto Credi, Andrea Secchi, Arturo Arduini
Published online: 9 January 2017
2017, Vol. 6, pp. 64-72
DOI: 10.1002/open.201600128
Tris‐(N ‐phenylureido)‐calix[6]arene derivatives are heteroditopic non‐symmetric molecular hosts that can form pseudorotaxane complexes with 4,4′‐bipyridinium‐type guests. Owing to the unique structural features and recognition properties of the calix[6]arene wheel, these systems are of interest for the design and synthesis of novel molecular devices and machines. We envisaged that the incorporation of photoactive units in the calixarene skeleton could lead to the development of systems the working modes of which can be governed and monitored by means of light‐activated processes. Here, we report on the synthesis, structural characterization, and spectroscopic, photophysical, and electrochemical investigation of two calix[6]arene wheels decorated with three naphthyl groups anchored to either the upper or lower rim of the phenylureido calixarene platform. We found that the naphthyl units interact mutually and with the calixarene skeleton in a different fashion in the two compounds, which thus exhibit a markedly distinct photophysical behavior. For both hosts, the inclusion of a 4,4′ ‐bipyridinium guest activates energy‐ and/or electron‐transfer processes that lead to non‐trivial luminescence changes.
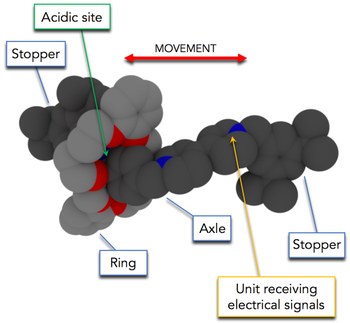
Remote electrochemical modulation of pKain a rotaxane by co-conformational allostery
Proceedings of the National Academy of Sciences of the United States of America
Giulio Ragazzon, Christian Schäfer, Paola Franchi, Serena Silvi, Benoit Colasson, Marco Lucarini, Alberto Credi
Published online: 27 November 2017
2018, Vol. 115, pp. 9385-9390
DOI: 10.1073/pnas.1712783115
Allosteric control, one of Nature’s most effective ways to regulate functions in biomolecular machinery, involves the transfer of information between distant sites. The mechanistic details of such a transfer are still an object of intensive investigation and debate, and the idea that intramolecular communication could be enabled by dynamic processes is gaining attention as a complement to traditional explanations. Mechanically interlocked molecules, owing to the particular kind of connection between their components and the resulting dynamic behavior, are attractive systems to investigate allosteric mechanisms and exploit them to develop functionalities with artificial species. We show that the pKa of an ammonium site located on the axle component of a [2]rotaxane can be reversibly modulated by changing the affinity of a remote recognition site for the interlocked crown ether ring through electrochemical stimulation. The use of a reversible ternary redox switch enables us to set the pKa to three different values, encompassing more than seven units. Our results demonstrate that in the axle the two sites do not communicate, and that in the rotaxane the transfer of information between them is made possible by the shuttling of the ring, that is, by a dynamic intramolecular process. The investigated coupling of electron- and proton-transfer reactions is reminiscent of the operation of the protein complex I of the respiratory chain.
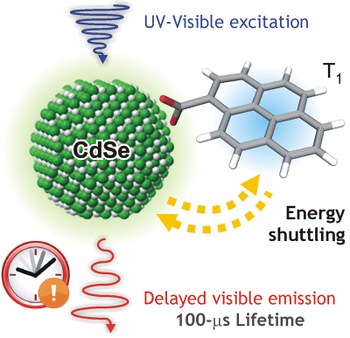
Designed long lived emission from CdSe quantum dots by reversible electronic energy transfer with a surface bound chromophore
Angewandte Chemie International Edition
Marcello La Rosa, Sergey A. Denisov, Gediminas Jonusauskas, Nathan D. McClenaghan, Alberto Credi
Published online: 2 February 2018
2018, Vol. 57, pp. 3104-3107
DOI: 10.1002/anie.201712403
The size-tunable emission of luminescent quantum dots (QDs) makes them highly interesting for applications that range from bioimaging to optoelectronics. For the same applications, engineering their luminescence lifetime – in particular, making it longer – would be as important; however, no rational methodology to reach this goal is available to date. Here we describe a strategy to prolong the emission lifetime of QDs by electronic energy shuttling to the triplet excited state of a surface-bound molecular chromophore. To implement this idea we made CdSe QDs of different sizes, and we self-assembled them with a pyrene derivative. We observed that the conjugates exhibit delayed luminescence, with emission decays that are prolonged by more than 3 orders of magnitude compared to the parent CdSe QDs (lifetimes up to 330 us). The mechanism invokes unprecedented reversible quantum dot-to-organic chromophore electronic energy transfer.