As part of the Photochemical Nanosciences Laboratory, established by Vincenzo Balzani, the research activity of the group is focused on photoactive supramolecular systems and nanoparticles. Quantum dots, dendrimers and luminescent or photochromic species are investigated for energy conversion (luminescent solar concentrators, artificial photosynthesis and photocatalysis), as well as imaging and photocontrolled nanostructures.
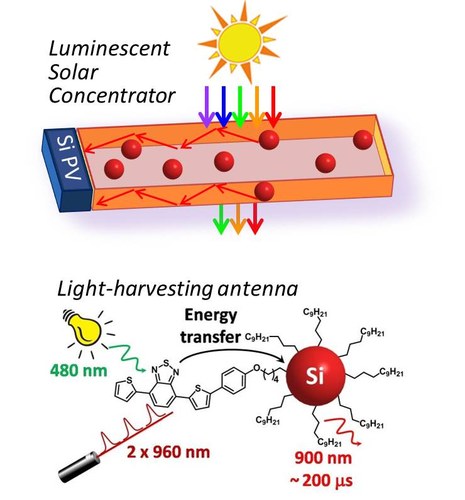
Light-harvesting antennae, constituted by silicon nanocrystals (SiNCs) covalently functionalized at their surface with chromophores display strong, tunable and long-lived (microsecond time-scale) luminescence, insensitive to dioxygen (PhysChemChemPhys 2017, 19, 26507 – 26526).
This research is funded by an ERC Starting Grant (PhotoSi) and ERC Proof of Concept (SiNbioSys) with two applications:
by luminescent solar concentrators (LSC) taking advantage of the apparent large Stokes shift of SiNCs, which absorb in the UV and emit in the red (diameter ca. 3 nm) to near-infrared (diameter ca. 5 nm) spectral region.
by time-gated detection, taking advantage of the long-lived emission of SiNCs (Nanoscale 2020, 12, 7921–7926). A step further is represented by the use of 2-photon absorbing chromophores integrated in SiNC light-harvesting antenna (Chem 2017, 2, 550 – 560).
Photocatalytic processes can not only change reaction rates, but also modify chemical equilibria and, particularly, convert light into chemical energy (Angew. Chem. Int. Ed. 2015, 54, 11320–11337). Our attention is devoted to the design of the photocatalysts for two main applications:
The aim of this research is: (i) the design of photocatalysts that are strong light-absorber in the visible region, photostable and with proper redox properties and (ii) the understanding of the photocatalytic mechanism. A recent example is the nickel-mediated enantioselective photoredox allylation of aldehydes (Angew. Chem. Int. Ed. 2022, 61, e202114981). This research is funded by an ITN grant (PhotoReAct).
from widely available raw materials, as H2O and CO2. Recent examples are the photoreduction of CO2 promoted by organic photosensitisers and a Mn(I) catalyst (Sust. Energ. Fuels 2023, 7, 3454) and the photoelectrochemical valorisation of biomass derivatives (Solar RRL 2023, 2300205).
This research is funded by a H2020 RIA project (CONDOR).
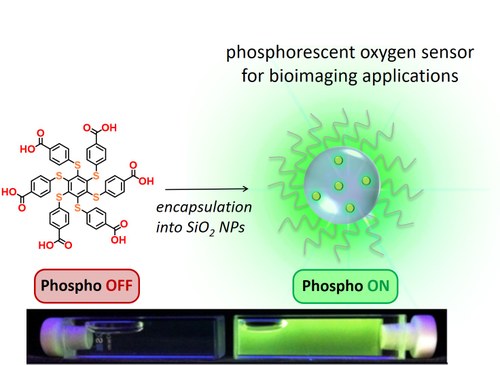
The hexathiobenzene core is not luminescent in fluid solution and becomes strongly phosphorescent (Fem ca. 1) in rigid media at room temperature (AIE chromophore, J. Mater. Chem. C 2013, 1, 2717).
Materials displaying room temperature phosphorescence hold promises as OLED emitters and sensors with time-gated detection. In a recent example, the hexathiobenzene group is functionalized by 6 terpyridine units: upon complexation of Mg2+ cations, a supramolecular polymer is formed and a strong phosphorescence is visible by naked eyes. The system performs as a sensor of Mg2+ cations and a very efficient light-harvesting antenna, which can be disassembled upon addition of fluoride anions (J. Am. Chem. Soc. 2014, 136, 6395).
The hexathiobenzene chromophore can also be encapsulated into silica nanoparticles for dioxygen sensing in bioimagin applications (J. Phys. Chem. C 2019, 123, 29884−29890).
This research is funded by the Italian-French University (PhD in cotutelle with Prof. Marc Gingras, Marseille).