Research
A brief description of our main research interests
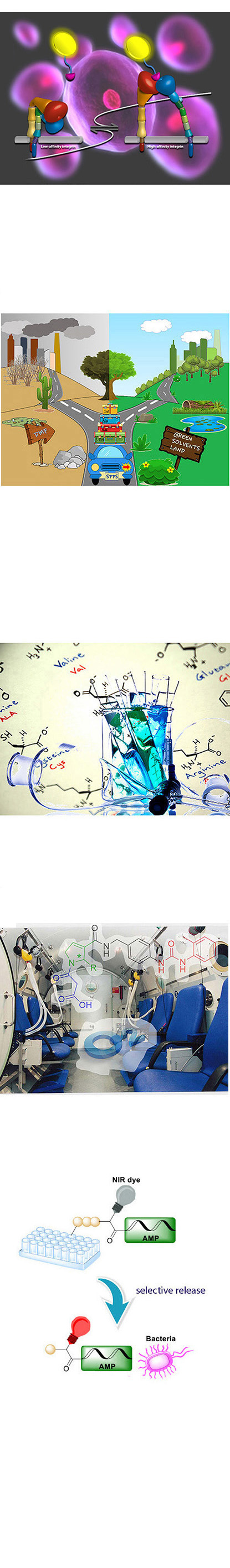
Design and synthesis of alpha-v-beta-3 and alpha-5-beta-1 integrin ligand conjugates as nanotheranostics for cancer cells detection and therapy
Weak protein-protein interactions are the widest occurring biological events regulating numerous functions. For this reason, the development of small molecules able to influence this network may afford useful tools for therapy and disclose information for the complete mapping of the interactome network. The group is interested in the interaction between integrins, transmembrane glycoproteins, and their extracellular matrix proteic partners. Due to the overexpression of alpha-v-beta-3 and alpha-5-beta-1 integrins on the surface of specific cancer cells, the design and synthesis of their ligands may be used to obtain delivery systems for cancer diagnosis and/or therapy. The design and synthesis of selective ligands has been already performed by mimicking the recognition sequence of integrin receptors, affording selective agonists and antagonist to these receptors.
Green Solid Phase Peptide Synthesis (SPPS) of oligopeptides for biological applications
Considering the increasing demand from the chemical and pharmaceutical markets for synthetic peptides, the attention to the optimization of SPPS protocols and their environmental impact is a relevant challenge. For instance, classic SPPS requires a large amount of solvent during the entire synthetic process and DMF is the most employed solvent, being however a highly reprotoxic solvent. The solvent has to efficiently assist the swelling of the resins, the couplings, the deprotections and the washings. A single solvent that is able to simultaneously afford optimal results during all these different steps may be difficult to find and for this reason a great number of green solvents are not suitable. Mixtures of different solvents showing efficient properties as swelling agents and solubilization media could represent novel successful tools, displaying better chemical/physical features than those exhibited by single components. The group is currently involved in the exploration of greener SPPS conditions and in the application of these novel approaches to pharmaceutical grade peptides.
Novel stereoselective methods for linear and cyclic-beta-amino acid synthesis as building block for bioactive peptidomimetics
The synthesis of bioactive peptidomimetics requires the development of novel methodologies for the preparation of enantiopure building blocks. To this purpose, novel protocols involving biocatalysts, organocatalysts, organic electrochemistry and traditional organometallic catalysts are developed for the preparation of b-amino acids and five membered heterocycles. These fragments are introduced into peptidomimetic structures, with the aim to induce conformational restraints and to reduce sensitivity to proteases. Moreover, on the basis of the fundamental requirements for protein-protein interaction, the design and synthesis of peptidomimetic compounds based on heterocyclic scaffolds is realized by introducing linear or cyclic b-amino acids into oligopeptides, with the aim to increase resistance to enzymatic digestion.
Design and synthesis of selective alpha-4-beta-1 and beta-2 integrin ligands as therapeutic tools for inflammatory diseases
Neutrophils, activated in case of acute or chronic inflammation, overexpress a large number of receptor for recognition and migration to the site on injury. Among them alpha-4-beta-1 and beta-2 integrins have a fundamental role. The goal of the project is the design and synthesis of peptidomimetic ligands for the subclasses of receptors that are overexpressed on leukocytes. Moreover, the combined activity of bioactive synthetic ligands with other therapeutic tools, as for instance hyperbaric oxygen treatment, is studied to propose novel approaches for inflammatory diseases, non-healing ulcers and for fibromyalgia.
Antibiotic biomaterials as new tools for rapid infection mapping and treatments in wound healing
The project has the goal of developing a simple, portable and economical tool for the treatment and identification, even in the preliminary phase, of infected chronic wounds. The construction of a multilayered biomaterial, obtained from recycled materials of the food industry, and of an antimicrobial peptide (AMP) conjugated to a molecule visible in the near infrared (Tag-NIR) has been undertaken. Each layer of the structures will be made up of different blends biopolymers with different levels cross-linking in order to have selected behavior and to be able to release the NIR molecule adsorbed inside it in a controlled manner. The realization of this project involved the Institute of Science and Technology of Ceramic Materials of Faenza (CNR-ISTEC) for the study of the biomaterial and the University of Bologna for the development of the functionalized peptide and for the first tests of antibiotic activity. The possibility to apply this treatment to patients affected by chronic wounds will be explored in collaboration with the Hyperbaric Centre of Ravenna. The project has been funded by Fondazione del Monte di Bologna e Ravenna
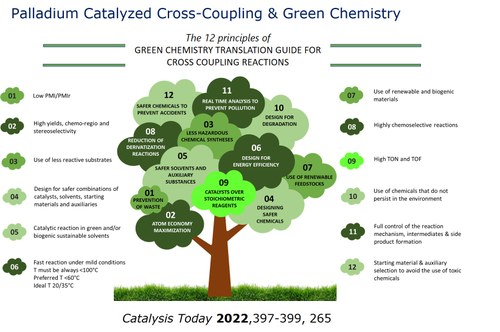
Development of cross-coupling methodologies under sustainable conditions. Heck-Cassar-Sonogashira and Suzuki reactions with low palladium loading in green chemistry. Reaction mechanism investigationsì.