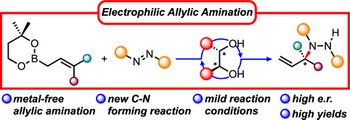
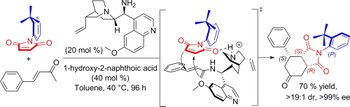
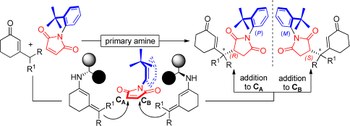
The asymmetric electrophilic amination of allylic boronates is realized using a chiral diol as a catalyst. This simple catalyst promotes the transfer of the allylic pendant to the activated N=N double bond of azodicarboxylate, leading to a series of enantioenriched hydrazides. The key aspect of the reaction mechanism is the ability of the chiral diol to form a chiral boronic ester that is more reactive than the achiral precursor, suppressing the racemic background reaction in favor of the enantioselective pathway. The reaction conditions ensure a rapid catalyst turnover, thus overcoming the typical rate-limiting step observed in previous allyl transfer reactions of boronic esters.