2017
Anionic Cyclometalated Iridium(III) Complexes with a Bis-Tetrazolate Ancillary Ligand for Light-Emitting Electrochemical Cells
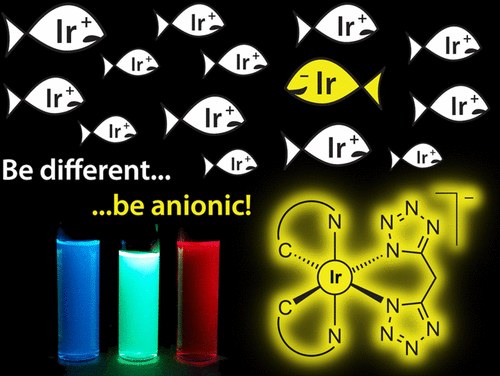
Anionic Cyclometalated Iridium(III) Complexes with a Bis-Tetrazolate Ancillary Ligand for Light-Emitting Electrochemical Cells
E. Matteucci, A. Baschieri, A. Mazzanti, L. Sambri, J. Ávila, A. Pertegás, H. J. Bolink, F. Monti, E. Leoni, N. Armaroli
Inorg. Chem. 2017, 56, 10584−10595
Betti’s base for crystallization-induced deracemization of substituted aldehydes: synthesis of enantiopure amorolfine and fenpropimorph
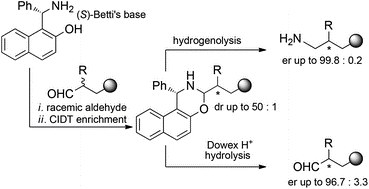
Betti’s base for crystallization-induced deracemization of substituted aldehydes: synthesis of enantiopure amorolfine and fenpropimorph
A. Carella, G. R. Ferronatto, E. Marotta, A. Mazzanti, P. Righi, C. Paolucci
Org. Biomol. Chem., 2017, 15, 2968
Conformational Analysis and Absolute Configuration of Axially Chiral 1‑Aryl and 1,3-Bisaryl-xanthines
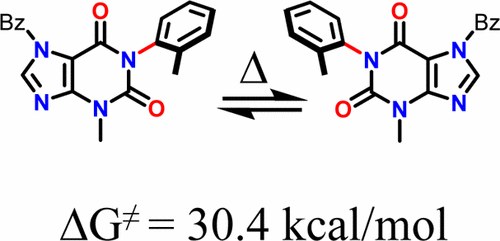
Conformational Analysis and Absolute Configuration of Axially Chiral 1‑Aryl and 1,3-Bisaryl-xanthines
M. Mancinelli, S. Perticarari, L. Prati, A. Mazzanti
J. Org. Chem. 2017, 82, 6874−6885
Controlling the C(sp3)−C(sp2) Axial Conformation in the Enantioselective Friedel−Crafts-Type Alkylation of β‑Naphthols with Inden-1-ones
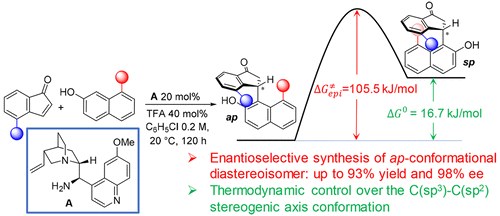
Controlling the C(sp3)−C(sp2) Axial Conformation in the Enantioselective Friedel−Crafts-Type Alkylation of β‑Naphthols with Inden-1-ones
N. Di Iorio, G. Filippini, A. Mazzanti, P. Righi, G. Bencivenni
Org. Lett. 2017, 19, 6692−6695
Highly Enantioselective Synthesis of Alkylpyridine Derivativesthrough a Michael/Michael/Aldol Cascade Reaction
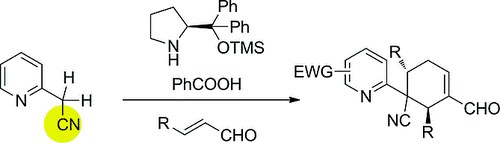
Highly Enantioselective Synthesis of Alkylpyridine Derivativesthrough a Michael/Michael/Aldol Cascade Reaction
M. Meazza, M. Potter, M. B. Pitak, S. J. Coles, A. Mazzanti, R. Rios
Eur. J. Org. Chem. 2017, 719–725
Hydroxy‐ and Methoxybenzene Derivatives with Benzenediazonium Salts ― Chemical Behavior and Tautomeric Problems
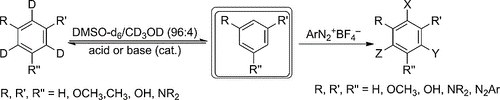
Hydroxy‐ and Methoxybenzene Derivatives with Benzenediazonium Salts ― Chemical Behavior and Tautomeric Problems
G. Micheletti, C. Boga, L. Forlani, E. Del Vecchio, N. Zanna, A. Mazzanti, M. Monari
Eur. J. Org. Chem. 2017, 964–974
Michael Addition of Oxindoles to N-(2-tert-Butylphenyl)maleimides: Efficient Desymmetrization for the Synthesis of Atropisomeric Succinimides with Quaternary and Tertiary Stereocenters
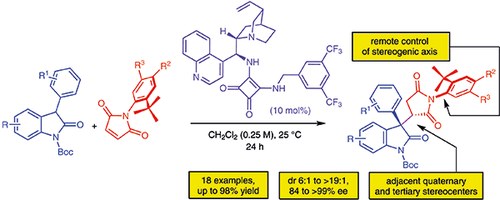
Michael Addition of Oxindoles to N-(2-tert-Butylphenyl)maleimides: Efficient Desymmetrization for the Synthesis of Atropisomeric Succinimides with Quaternary and Tertiary Stereocenters
N. Di Iorio, L. Soprani, S. Crotti, E. Marotta, A. Mazzanti, P. Righi, G. Bencivenni
Synthesis 2017, 49, 1519–1530
http://dx.doi.org/10.1055/s-0036-1588408
N-heterocyclic linkers from 1,2-diaza-1,3-dienes for dye-sensitizedsolar cells: DFT calculations, synthesis and photovoltaic performance
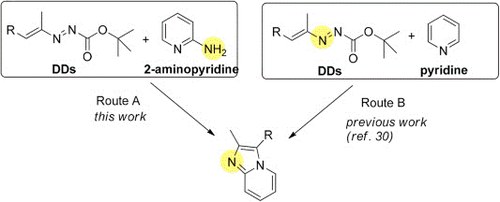
N-heterocyclic linkers from 1,2-diaza-1,3-dienes for dye-sensitizedsolar cells: DFT calculations, synthesis and photovoltaic performance
A. Fattori, R. Majer, A. Mazzanti, M. F. Ottaviani, A. Modelli, F. Mantellini, S. Santeusanio
Dyes and Pigments 2017, 145, 246-255
Nucleophilic Dearomatization of Pyridines under Enamine Catalysis: Regio-, Diastereo-, and Enantioselective Addition of Aldehydes to Activated N-Alkylpyridinium Salts
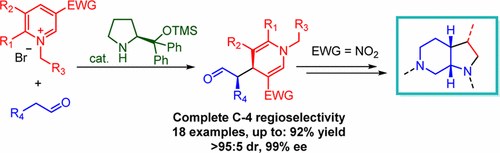
Nucleophilic Dearomatization of Pyridines under Enamine Catalysis: Regio-, Diastereo-, and Enantioselective Addition of Aldehydes to Activated N-Alkylpyridinium Salts
G. Bertuzzi, A. Sinisi, D. Pecorari, L. Caruana, A. Mazzanti, L. Bernardi, M. Fochi
Org. Lett. 2017, 19, 834−837
Organocatalytic Asymmetric Sulfa‐Michael Addition of 2‐Aminothiophenols to Chalcones: First Enantioselective Access to 2,3,4,5‐Tetrahydro‐1,5‐benzothiazepines
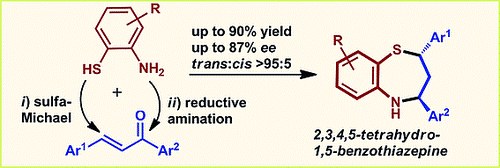
Organocatalytic Asymmetric Sulfa‐Michael Addition of 2‐Aminothiophenols to Chalcones: First Enantioselective Access to 2,3,4,5‐Tetrahydro‐1,5‐benzothiazepines
V. Corti, P. Camarero Gonzalez, J. Febvay, L. Caruana, A. Mazzanti, M. Fochi, L. Bernardi
Eur. J. Org. Chem. 2017, 49–52
Synthesis and investigation on processing-depending polarized fluorescence emission in thin-films of 2,2′-([2,2′-bithiophene]-5,5′-diyl)bis(5-octyl-4-phenyl-4H-thieno[2,3-c]pyrrol-6(5H)-one)
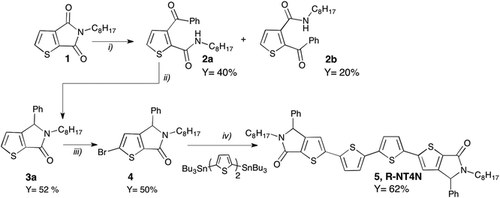
Synthesis and investigation on processing-depending polarized fluorescence emission in thin-films of 2,2′-([2,2′-bithiophene]-5,5′-diyl)bis(5-octyl-4-phenyl-4H-thieno[2,3-c]pyrrol-6(5H)-one)
L. Favaretto, M. Zambianchi, S. G. Lopez, A. Mazzanti, C. Zanardi, R. Seeber, D. Gentili, F. Valle, E. Benvenuti, M. Muccini, G. Ruani, F. Mercuri, S. Milita, F. Liscio, M. Cavallini, S. Toffanin, M. Melucci
J. Mater. Chem. C, 2017, 5, 10320
Tetrasubstituted cyclopentadienones as suitable enantiopure ligands with axial chirality
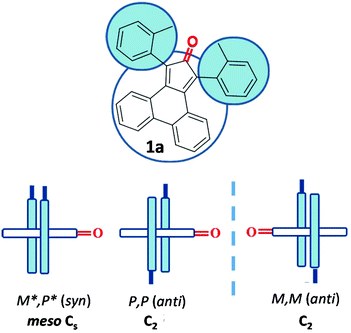
Tetrasubstituted cyclopentadienones as suitable enantiopure ligands with axial chirality
L. Prati, M. Mancinelli, A. Ciogli, A. Mazzanti
Org. Biomol. Chem., 2017, 15, 8720
3,5-Dinitrobenzoyl-9-amino-9-deoxy-9-epiquinine as Pirkle-Anion Exchange Hybrid-Type Chiral Selector in High-Performance Liquid Chromatography
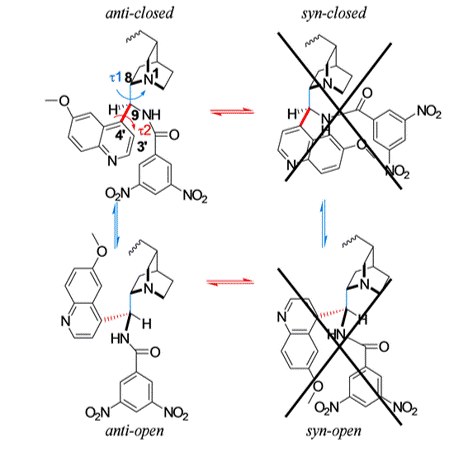
3,5-Dinitrobenzoyl-9-amino-9-deoxy-9-epiquinine as Pirkle-Anion Exchange Hybrid-Type Chiral Selector in High-Performance Liquid Chromatography
M. De Martino, G. Bencivenni, A. Mazzanti, S. Menta, O. H. Ismail, R. Sabia, A. Ciogli
Chromatographia 2017, 80, 751–762
Enantioselective Approaches to 3,4-Annulated Indoles Using Organocatalytic Domino Reactions
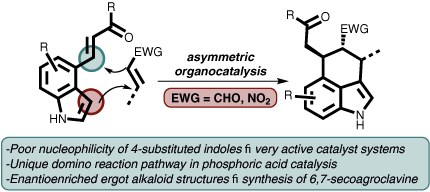
Enantioselective Approaches to 3,4-Annulated Indoles Using Organocatalytic Domino Reactions
Lorenzo Caruana, Mariafrancesca Fochi and Luca Bernardi
Synlett 2017, 28, 1530–1543
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0036-1589494