Projects
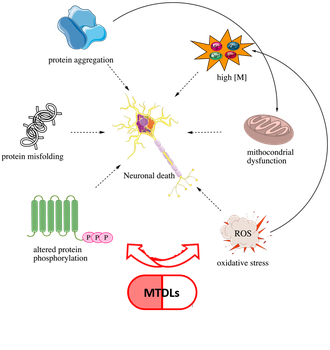
MTDLs FOR NEURODEGENERATIVE DISEASES
The development of multi-target-directed ligands (MTDLs) is a polypharmacological strategy that is emerging as a successful option for the development of potential drug candidates for neurodegenerative diseases. Neurodegeneration is a complex and multifactorial process that involves various, interconnected molecular pathways that lead to progressive and irreversible deterioration in CNS functions. Therefore, our goal is to design and synthesize MTDLs able to modulate simultaneously different targets involved in neurodegeneration onset and progression (see our J. Med. Chem. “Multi-target-directed ligands to combat neurodegenerative diseases” Perspective, ranked by ISI as a “highly cited paper”).
In particular, in the neurodegenerative field, our aim is to design and develop MTDLs to treat Alzheimer’s Disease (AD). AD is, to date, the major cause of dementia worldwide and the number of affected patients is expected to increase by 2050. Currently, on the market, there are only palliative drugs. Notwithstanding massive investments in basic and translational research by government and non-profit organizations worldwide, pharmaceutical companies continue to view AD drug discovery as an extremely risky area. For this reason, it is an imperative responsibility of the academic sector to ensure basic research in this field in order to contribute to finding an effective cure against AD and better address this unmet medical need.
DEVELOPMENT OF BIFUNCTIONAL SMALL MOLECULES:
1) PROTACs FOR NEGLECTED DISEASES
The multi-target approach can be a successful tool when applied to NTDs because these drugs are generally safer and more efficient, thanks to the synergism on different targets. Moreover, the development of bifunctional molecules could also be a possible approach to deal with the emergency of new resistant strains and important side effects. All these concepts were enlightened in J. Med. Chem. 2009, 52, 23, 7339–7359.
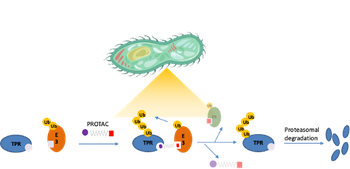
Recently, we have turned our attention to the development of another class of innovative bifunctional compounds, i.e., PRoteolysis TArgeting Chimeras (PROTACs) for leishmaniasis. PROTACs are heterobifunctional small molecules featuring a ligand for the protein of interest (POI), and a ligand that recruits an E3 ubiquitin ligase connected via a suitable linker. The PROTAC induces a ternary complex formation between the POI and E3 ligase, which leads to POI degradation by the proteasome. As such, PROTAC-mediated degradation is a revolutionary therapeutic modality, which is driving not only a radical rethink of small molecule-based therapeutics, but also a truly drug discovery transition (as discussed in our recent perspective J. Med. Chem. 2022, 65, 14, 9507–9530).
Neglected Tropical Diseases (NTDs) are a heterogeneous group of highly debilitating infections (i.e. Leishmaniasis, African trypanosomiasis, Chagas Disease) that affect mainly the tropical and subtropical areas. These diseases can be caused by bacteria, viruses, helminth, and protozoa. The most affected communities are the ones living in poor conditions because of the restricted access to basic sanitation, the proximity to animals and livestock and thus to vectors of these infective diseases. However, following changing climate conditions and migratory flows, leishmaniasis is today an emerging disease also in many countries in the Mediterranean basin. Toxicity and development of resistance are critical issues of current treatments (i.e. amphotericin B and miltefosin).
Already successfully applied to oncology and viral/bacterial infections, among others, no PROTACs for leishmaniasis and other neglected diseases have been reported. Despite many challenges ahead, PROTAC-mediated protein degradation for leishmaniasis may lead to the development of next-generation leishmanicidal drugs (ACS Bio Med Chem Au 2023, 3, 1, 32–45).
2) PURSUING THE PROTAC APPROACH TO COMBAT EMERGING FLAVIVIRUS INFECTIONS
Flaviviruses are vector-borne RNA viruses causing severe diseases, including epidemics of dengue and West Nile Virus (WNV), and the recent outbreak of Zika virus (Pierson, T. C.; Diamond, M. S. Nat Microbiol. 2020, 5(6): 796–812). Measure to control and cure flavivirus-induced infections are largely underestimated. To mitigate current health burden and limit future outbreaks, drugmakers need to be ready and avoid that the spread of flaviviruses will materialize, in the worst-case scenario, in a pandemic. Currently, there is neither vaccine nor drug to treat flavivirus infection, making drug-discovery efforts extremely urgent. Despite different families of small molecules or peptidomimetic inhibitors have been reported, none of them reached clinical phases (Voss, S.; Nitsche, C. RSC Med. Chem., 2021, 12, 1262).
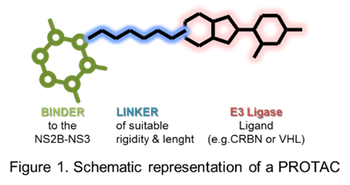
Here, as game-changer, we are proposing the proteolysis targeting chimeras (PROTAC) approach to address Flavivirus infections through the targeting of the NS2B-NS3 protease (Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer M., Pheng Lim, S.; Yin, Z.; Keller H. T.; Vasudevan S. G; Hommel U. Nat. Struct. Mol. 2006, 13, 373). PROTAC are heterobifunctional molecules with the ability to harness ubiquitination and subsequent proteasomal degradation, by putting in close proximity the protein of interest (POI) and an E3 ligase. In fact, PROTAC are structurally constituted of a ligand, directed to the POI, bridged to an E3 ligase by a suitable linker ((a) Salerno, A.; Seghetti, F.; Caciolla, J.; Uliassi, E.; Testi, E.; Guardigni, M.; Roberti, M.; Milelli, A.; Bolognesi, M. L. J. Med. Chem. 2022, 65, 9507−9530. b) Espinoza-Chávez, R. M.; Salerno, A., Liuzzi, A.; Ilari, A.; Milelli, A.; Uliassi E.; Bolognesi, M. L. ACS Bio Med Chem Au 2023, 3, 1, 32–45).
The NS2B-NS3 protease is essential for the replication of flaviviruses and, importantly, is conserved among WNV, Dengue and Zika viruses. Therefore, our final PROTACs should possess pan-flaviviral activity and might represent a new avenue to target flaviviruses.
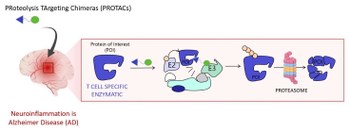
3) PROTACs DIRECTED TO NEUROINFLAMMATORY TARGETS
Increasing evidence suggests that Alzheimer Disease (AD) pathogenesis is a complex multifactorial event, which includes β-amyloid aggregation, neurofibrillary tangle formation, and neuroinflammation (Genome Med. 2023;15(1):6. Published 2023 Jan 26). Neuroinflammation is one of the cardinal features of AD and refers to a brain specific and chronic inflammation-like response that leads to neuronal death. Central and peripheral immune systems trigger an innate immune response characterized by release of inflammatory mediators, which contribute to AD progression and severity. Thus, modulating crucial neuroinflammatory targets is a strategy under intensive investigation in AD drug discovery. On this basis, in this project, we have turned our attention to a revolutionary therapeutic modality, i.e., PRoteolysis TArgeting Chimeras (PROTACs) for key neuroinflammatory targets. PROTACs are heterobifunctional small molecules featuring a ligand for the protein of interest (POI), in our case an anti-neuroinflammatory agent active on T cell specific enzymatic, and a ligand that recruits an E3 ubiquitin ligase connected via a suitable linker. PROTAC induces a ternary complex formation between the POI and E3 ligase, which leads to POI degradation by the proteasome (Chem Soc Rev. 2022;51(12):5214-5236).
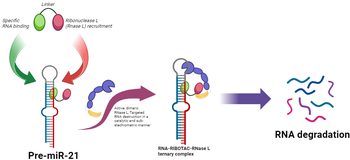
4) TRIGGERING pre-miRNA21 DEGRADATION BY RIBONUCLEASE TARGETING CHIMERAS (RIBOTACs)
Nowadays, RNA is a very hot topic, indeed, one of the major challenges of current medicinal chemistry is targeting noncoding RNAs using small-molecule drugs. Currently, RNA-based marketed drugs show limitations, including ASOs, silent RNA and CRISPR cas9 gene editing, such as poor cellular uptake, low tissue specificity, increase hospitalization and in worst cases toxicity.
Small Molecules represent the promising way to development pharmaceutical drugs in the future. One of the most promising strategies is represented by RIBOTACs. This because small molecules and lead medicines are able of taking advantage of available binding sites built by the rearrangement of RNA into secondary and tertiary structures, and as complex with RNA binding proteins (RBPs) (Hargrove, A. E. Chem Commun 2020 56, 14744-14756).
Thanks to the NRRP (National Recovery and Resilience Plan), this project aims to develop ribonuclease targeting chimeras (RIBOTACs) to target pre-miRNA21, a well-known protooncogene, marker of fibrosis, and molecular link between inflammation and various diseases. RIBOTACs are bifunctional small molecules that are designed to trigger the degradation of ncRNAs through the activation of RNase L nuclease. Simultaneously, RIBOTACs comprises an RNA-binder, called warhead, conjugated by a linker to another small molecule that recruits and locally activates RNase L, makes dimerization, and induce the enzymatic cleavage of an RNA of Interest (ROI). We need the formation of a ternary complex between ROI-RIBOTAC-RNase L which induces the consequent selective degradation of the ROI. Therefore, RIBOTAC technology has the potential to open new avenues in research into physiological and disease-associated mechanisms and lay the foundations for potential treatments of severe conditions (Childs-Disney, J. L. et al. Nature Reviews Drug Discovery 2022 21, 736-762).
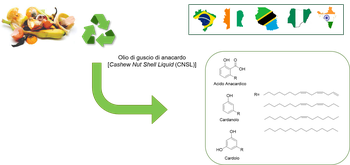
SUSTAINABLE DRUG DISCOVERY
The research and development of new potential drug candidates require huge expenses and efforts which are eventually mirrored in the final price of the drugs on the market. Access to medicines, including availability and affordability is a major global public health threat for all the therapeutic areas. It is a matter of special concern for those diseases affecting low-income populations (i.e. poverty-related disease as NTD) but also for Alzheimer’s Disease, which is expected to affect large populations in the developing countries. Through a Sustainable Drug Discovery approach, we aim to develop potential treatments that are affordable and globally accessible. In this context, an enlightening possibility is represented by the valorization of food byproduct material (Chem. Soc. Rev., 2021, 50, 11191-11207), such as the cashew-nutshell liquid (CNSL). CNSL components (i.e. anacardic acid, cardanol, cardol) are bioactive phenolic compounds with a linear chain of different unsaturation degrees. Thanks to their peculiar amphiphilic structure and their innate biological activities, CNSL components are particularly versatile for the functionalization and the design of MTDLs for diverse diseases (e.g., ChemMedChem 2019, 14, 621 and J. Med. Chem. 2021, 64, 4972−499).
How to reach us
Contatti
Ph.D. Student Office
+39 051 20 9 9717