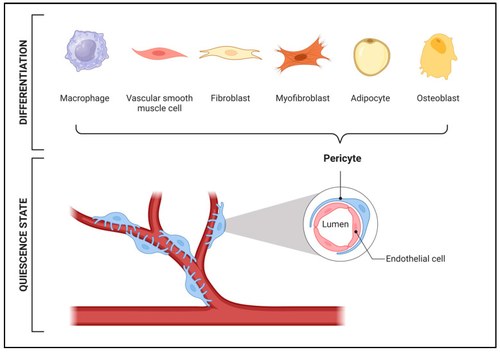
Collagen VI (COLVI) Deficiency Myopathies (COLVI-RM) are rare genetic diseases caused by COLVI mutations that lead to altered secretion of COLVI and, consequently, a disruption of the integrity of the extracellular matrix (ECM). Affected patients show muscle weakness, connective tissue abnormalities, and a progressive reduction in muscle fibers. Indeed, COLVI deficiency alters the structure and biomechanical properties of the ECM, also affecting muscle regeneration and causing clinical symptoms of varying severity. Despite the known implications, the molecular and cellular mechanisms underlying muscle atrophy remain unclear. Recently, it has been demonstrated that pericytes, mesenchymal stem cells closely associated with endothelial cells in blood vessels, play a crucial role in muscle regeneration. Under physiological conditions, pericytes remain in a quiescent state and become active only under specific circumstances. For instance, in response to muscle injury or strong hormonal stimuli, pericytes can differentiate into muscle-like cells, promoting faster regeneration of damaged muscle through the release of specific trophic factors. Preliminary data from muscle biopsies have shown significant alterations in the number and distribution of pericytes around blood capillaries in COLVI-RM patient samples compared to healthy donor samples. Specifically, a reduction in pericyte coverage of blood vessels and an increase in capillary basement membrane thickness were observed exclusively in COLVI-RM patient biopsies. These preliminary findings suggest a potential involvement of pericytes in the pathogenesis of COLVI-RM. This project explores the role of pericytes in COLVI-RM, focusing on cellular and molecular mechanisms in affected patients compared to healthy individuals. Furthermore, it investigates the effects of COLVI binding alterations with its membrane receptor Neural/Glial Antigen 2 (NG2), in relation to pericyte proliferation and quiescence states. The completion of this study could represent a significant step toward understanding the pathogenesis of COLVI deficiency myopathies (COLVI-RM), with a particular focus on the mechanisms underlying muscle regeneration. The results could contribute to the understanding of the cellular and molecular mechanisms underlying COLVI-RM, ultimately paving the way for the development of potential therapeutic strategies, as no specific pharmacological treatment is currently available.