Milestones and Expected Outputs
Milestone 1: Requirement Analysis and Experimental Protocol definition [duration: m0-m6]
The milestone will involve all project partners in defining a set of linguistic and clinical requirements for upcoming project activities.
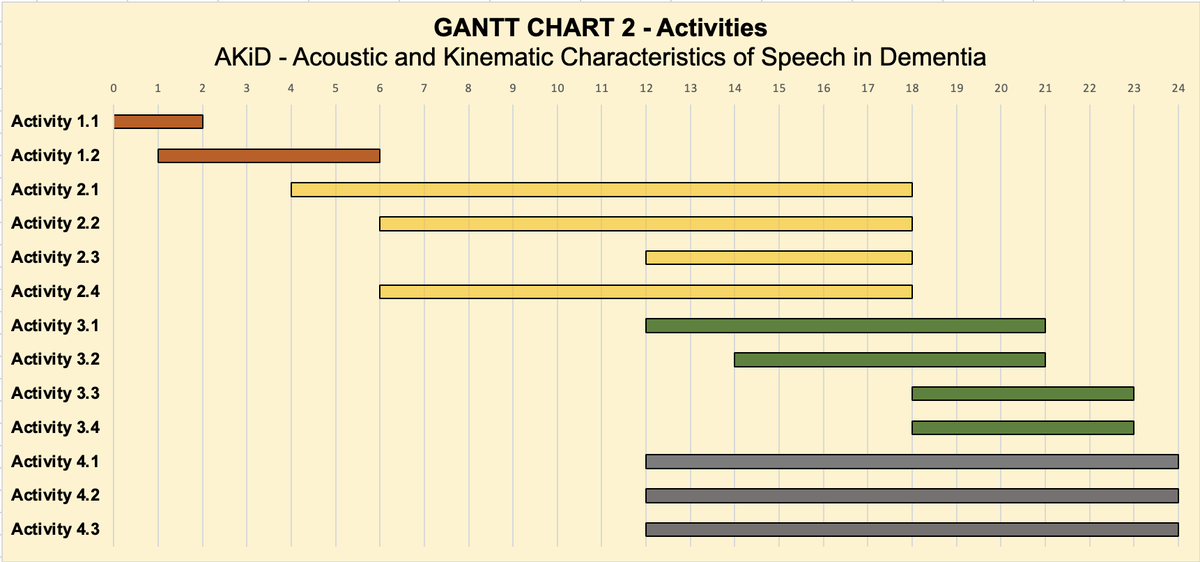
- Activity 1.1: Definition of the cohorts and data types [duration: m0-m2]
The activity will establish the standards for subject inclusion/exclusion in the clinical trials and the kinds of data that are expected (e.g., number of patients, stratification, and balancing of the cohort).
Outcome: Project document 1 - Official research protocol (§ Characteristics of the enrolled cohorts) - Activity 1.2: Creation of tasks [duration: m1-m6]
The task will supply the full battery of linguistic tasks to be administered. It will also specify the language materials for speech elicitation (i.e., input stimuli, reading texts, and questions).
Outcome: Project document 1 - Official research protocol (§ Speech elicitation strategies and experimental materials)
Milestone 2: Data collection [duration: m4-m18]
The milestone will center on gathering speech data from patients and healthy controls.
- Activity 2.1: Clinical screening/patients enrollment [duration: m4-m17]
The patients chosen following the criteria defined in A1.1 will be enrolled for A2.2 and A2.3.
Outcome: CRF - Case Report Form [AKiD_O_M2_A2_1-CRF] - Activity 2.2: Acoustic data acquisition [duration: m6-m18]
At least 40 patients (enrolled in A2.1.) will be recorded while the linguistics tasks (cf. A1.2) are being completed.
Outcome (with access limited to project partners): At least 7 minutes of reading and semi-spontaneous speech for each patient. - Activity 2.3: UTI data acquisition [duration: m12-m18]
At least 20 patients (enrolled in A2.1.) will undergo Ultrasound Tongue Imaging while performing the linguistic task defined in A1.2.
Outcome (with access limited to project partners): At least 5 minutes of reading for each patient, with 2 repetitions of the sentence-list task. - Activity 2.4: Control groups enrollment [duration: m6-m18]
Control speakers will be recorded both acoustically and articulatory.
Outcome (with access limited to project partners): at least 10 minutes of reading and semi-spontaneous speech for each control speaker in the acoustic recording. At least 5 minutes of reading with UTI for each control speaker, with 3 repetitions of the sentence-list task.
Milestone 3: Data annotation and analysis [duration: m12-m23]
The milestone will deal with the statistical analysis of the data obtained through M2, both qualitatively and quantitatively.
- Activity 3.1: Acoustic Data annotation [duration: m12-m21]
This annotation phase will include preliminary work in which the RUs will elaborate an annotation protocol for data transcription and annotation in PRAAT. The annotation of the “pathological” acoustic data will be carried out mainly at the UniMi RU, with the support of the contract researcher enrolled in the project. UniBo will be in charge of the control group.
The annotation will include: an orthographic time-aligned transcription of both controlled and semi-spontaneous speech; acoustic annotation of the target phonemes under analysis (i.e., stressed vowels, voiceless and voiced stops).
Outcome: Project document 2 - Research protocol for acoustic data annotation of pathological speech; creation of a transcribed and annotated acoustic corpus. - Activity 3.2: UTI Data annotation [duration: m14-m21]
After the annotation of the acoustic data associated with the UTI recordings (i.e., controlled speech), the data will be articulatory annotated by tracing in a semi-automatic way the tongue profile during the phonation of the target vowels and consonants in the two phonological contexts (singleton vs. geminate). This process will include a frame-by-frame check of each token in both the pathological subjects and the controlled group.
Outcome: Project document 3 - Research protocol for UTI data annotation of pathological speech; full annotated dataset. - Activity 3.3: Acoustic Data analysis [duration: m18-m23]
Qualitative and quantitative analysis of the data acquired in A2.2 and post-processed in A3.1.
Outcome: AKiD acoustic corpus, including annotated acoustic data (.Textgrid), tables of vowel formants’ values (.csv), and dynamic graphs (created with Visible Vowels, Heeringa & Van de Velde 2018). Statistical modeling and outputs produced in R will also be included, in line with FAIR data principles. - Activity 3.4: UTI Data analysis [duration: m18-m23]
Qualitative and quantitative analysis of the data acquired in A2.3 and post-processed in A3.2
Outcome: AKiD articulatory corpus, including graphs of tongue profiling and tables (.csv) of articulatory values such as Anticipatory movement and velocity. Statistical modeling and outputs produced in R will also be included, in line with FAIR data principles.
Milestone 4: Scientific Dissemination [duration: m12-m24]
The milestone will aim at establishing a continual relationship with the scientific community, the clinical stakeholders, and the general public.
- Activity 4.1: Conferences
Presenting the project results to the scientific community by attending major national and international conferences.
Outcome: the Research Group will jointly attend at least two international conferences. At the end of the project, a final workshop will also be organized. - Activity 4.2: Scientific publications
Presenting the project results to the scientific community by submitting papers to major international journals.
Outcome: the Research Group will jointly submit at least two papers to major scientific venues. - Activity 4.3: Third Mission
Spread knowledge about the research topic and transferring research results outside of academic settings
Outcome: the Research Group will organize at least two Public Engagements activities: one public seminar (e.g. at the European Researcher's Night), and one article for the general public (e.g., for the internet site “Linguisticamente”).